Paclitaxel Prescribing Information
Paclitaxel Injection, USP should be administered under the supervision of a physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of complications is possible only when adequate diagnostic and treatment facilities are readily available.
Anaphylaxis and severe hypersensitivity reactions characterized by dyspnea and hypotension requiring treatment, angioedema, and generalized urticaria have occurred in 2% to 4% of patients receiving paclitaxel in clinical trials. Fatal reactions have occurred in patients despite premedication. All patients should be pretreated with corticosteroids, diphenhydramine, and H2 antagonists. (See
Paclitaxel therapy should not be given to patients with solid tumors who have baseline neutrophil counts of less than 1,500cells/mm3 and should not be given to patients with AIDS-related Kaposi’s sarcoma if the baseline neutrophil count is lessthan 1,000 cells/mm3. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving paclitaxel.
Paclitaxel Injection, USP is indicated as subsequent therapy for the treatment of advanced carcinoma of the ovary. As first-line therapy, paclitaxel is indicated in combination with cisplatin.
Paclitaxel is indicated for the adjuvant treatment of node-positive breast cancer administered sequentially to standard doxorubicin-containing combination chemotherapy. In the clinical trial, there was an overall favorable effect on disease-free and overall survival in the total population of patients with receptor-positive and receptor-negative tumors, but the benefit has been specifically demonstrated by available data (median follow-up 30 months) only in the patients with estrogen and progesterone receptornegative tumors. (See
Paclitaxel Injection, USP is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
Paclitaxel, in combination with cisplatin, is indicated for the first-line treatment of nonsmall cell lung cancer in patients who are not candidates for potentially curative surgery and/or radiation therapy.
Paclitaxel is indicated for the second-line treatment of AIDS-related Kaposi’s sarcoma.
All patients should be premedicated prior to paclitaxel administration in order to prevent severe hypersensitivity reactions. Such premedication may consist of dexamethasone 20 mg PO administered approximately 12 and 6 hours before paclitaxel, diphenhydramine (or its equivalent) 50 mg I.V. 30 to 60 minutes prior to paclitaxel, and cimetidine (300 mg) or ranitidine (50 mg) I.V. 30 to 60 minutes before paclitaxel.
For patients with carcinoma of the ovary the following regimen is recommended: (
1) For previously untreated patients with carcinoma of the ovary, one of the following recommended regimens may be given every 3 weeks. In selecting the appropriate regimen, differences in toxicities should be considered (see Table 11 in
a. Paclitaxel administered intravenously over 3 hours at a dose of 175 mg/m2 followed by cisplatin at a dose of 75 mg/m2; or
b. Paclitaxel administered intravenously over 24 hours at a dose of 135 mg/m2 followed by cisplatin at a dose of 75 mg/m2.
2) In patients previously treated with chemotherapy for carcinoma of the ovary, paclitaxel has been used at several doses and schedules; however, the optimal regimen is not yet clear. (See
For patients with
1) For the adjuvant treatment of node-positive breast cancer, the recommended regimen is paclitaxel, at a dose of 175 mg/m2 intravenously over 3 hours every 3 weeks for 4 courses administered sequentially to doxorubicin-containing combination chemotherapy. The clinical trial used 4 courses of doxorubicin and cyclophosphamide (see
2) After failure of initial chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy, paclitaxel at a dose of 175 mg/m2 administered intravenously over 3 hours every 3 weeks has been shown to be effective.
For patients with
For patients with
Based upon the immunosuppression in patients with advanced HIV disease, the following modifications are recommended in these patients:
1) Reduce the dose of dexamethasone as 1 of the 3 premedication drugs to 10 mg PO (instead of 20 mg PO);
2) Initiate or repeat treatment with paclitaxel only if the neutrophil count is at least 1,000 cells/mm3;
3) Reduce the dose of subsequent courses of paclitaxel by 20% for patients who experience severe neutropenia (neutrophil <500 cells/mm3 for a week or longer); and
4) Initiate concomitant hematopoietic growth factor (G-CSF) as clinically indicated.
For the therapy of patients with solid tumors (ovary, breast and NSCLC), courses of paclitaxel should not be repeated until the neutrophil count is at least 1,500 cells/mm3 and the platelet count is at least 100,000 cells/mm3. Paclitaxel should not be given to patients with AIDS-related Kaposi’s sarcoma if the baseline or subsequent neutrophil count is less than 1,000 cells/mm3. Patients who experience severe neutropenia (neutrophil <500 cells/mm3 for a week or longer) or severe peripheral neuropathy during Paclitaxel Injection, USP therapy should have dosage reduced by 20% for subsequent courses of paclitaxel. The incidence of neurotoxicity and the severity of neutropenia increase with dose.
Hepatic Impairment:
Degree of Hepatic Impairment | Recommended Paclitaxel Dosec | |||
Transaminase Levels | Bilirubin Levelsb | |||
24-Hour Infusion | ||||
| <2 x ULN | and | ≤1.5 mg/dL | 135 mg/m2 | |
| 2 to <10 x ULN | and | ≤1.5 mg/dL | 100 mg/m2 | |
| <10 x ULN | and | 1.6-7.5 mg/dL | 50 mg/m2 | |
| ≥10 x ULN | or | >7.5 mg/dL | Not recommended | |
3-Hour Infusion | ||||
| <10 x ULN | and | ≤1.25 x ULN | 175 mg/m2 | |
| <10 x ULN | and | 1.26-2.0 x ULN | 135 mg/m2 | |
| <10 x ULN | and | 2.01-5.0 x ULN | 90 mg/m2 | |
| ≥10 x ULN | or | >5.0 x ULN | Not recommended | |
| a These recommendations are based on dosages for patients without hepatic imairement of 135 mg/m2 over 24 hours or 175 mg/m over 3 hours; data are not available to make dose adjustment recommendations for other regimens (eg, for AIDS-related Kaposi’s sarcoma). b Differences in criteria for bilirubin levels between the 3- and 24-hour infusion are due to differences in clinical trial design. c Dosage recommendations are for the first course of therapy; further dose reduction in subsequent courses should be based on individual tolerance. | ||||
Given the possibility of extravasation, it is advisable to closely monitor the infusion site for possible infiltration during drug administration. (See
Upon preparation, solutions may show haziness, which is attributed to the formulation vehicle. No significant losses in potency have been noted following simulated delivery of the solution through I.V. tubing containing an in-line (0.22 micron) filter.
Data collected for the presence of the extractable plasticizer DEHP [di-(2-ethylhexyl) phthalate] show that levels increase with time and concentration when dilutions are prepared in PVC containers. Consequently, the use of plasticized PVC containers and administration sets is not recommended.
Paclitaxel solutions should be prepared and stored in glass, polypropylene, or polyolefin containers. Non-PVC containing administration sets, such as those which are polyethylene-lined, should be used.
Paclitaxel should be administered through an in-line filter with a microporous membrane not greater than 0.22 microns. Use of filter devices such as IVEX-2® filters which incorporate short inlet and outlet PVC-coated tubing has not resulted in significant leaching of DEHP.
The Chemo Dispensing Pin™ device or similar devices with spikes should not be used with vials of paclitaxel since they can cause the stopper to collapse resulting in loss of sterile integrity of the paclitaxel solution.
Paclitaxel Injection, USP is contraindicated in patients who have a history of hypersensitivity reactions to paclitaxel or other drugs formulated in Polyoxyl 35 Castor Oil, NF.
Paclitaxel Injection, USP should not be used in patients with solid tumors who have baseline neutrophil counts of <1,500 cells/mm3
or in patients with AIDS-related
kaposi’s sarcoma with baseline neutrophil counts of <1,000 cells/mm3.
| | Percent of Patients (n=812) |
|---|---|
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Leukopenia <4,000/mm3 <1,000/mm3 - Thrombocytopenia <100,000/mm3 <50,000 /mm3 - Anemia <11 g/dL <8 g/dL - Infections - Bleeding - Red Cell Transfusions - Platelet Transfusions | 90 52 90 17 20 7 78 16 30 14 25 2 |
| • Hypersensitivity Reactionb - All - Severe† | 41 2 |
• Cardiovascular - Vital Sign Changesc - Bradycardia (n=537) - Hypotension (n=532) - Significant Cardio vascular Events | 3 12 1 |
• Abnormal ECG - All Pts - Pts with normal baseline (n=559 ) | 23 14 |
• Peripheral Neuropathy - Any symptoms - Severe symptoms† | 60 3 |
• Myalgia/Arthralgia - Any symptoms- Severe symptoms† | 60 8 |
• Gastrointestinal - Nausea and vomiting - Diarrhea - Mucositis | 52 38 31 |
• Alopecia | 87 |
• Hepatic (Pts with normal baseline and on study data) - Bilirubin elevations (n=765) - Alkaline phosphatase elevations (n=575) - AST (SGOT) elevations (n=591) | 7 22 19 |
• Injection Site Reaction | 13 |
| a Based on worst course analysis. b All patients received premedication. c During the first 3 hours of infusion. † Severe events are defined as at least Grade III toxicity. | |
None of the observed toxicities were clearly influenced by age.
| | Percent of Patients | |||
Intergroup | GOG-111 | |||
T175/3b c75c (n=339) | C750c c75c (n=336) | T135/24b c75c (n=196) | C750c c75c (n=213) | |
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Thrombocytopenia <100,000/mm3e <50,000/mm3 - Anemia <11 g/dLf <8 g/dL - Infections - Febrile Neutropenia | 91d 33d 21d 3d 96 3d 25 4 | 95d 43d 33d 7d 97 8d 27 7 | 96 81d 26 10 88 13 21 15d | 92 58d 30 9 86 9 15 4d |
• Hypersensitivity Reaction - All - Severe† | 11d 1 | 6d 1 | 8d,g 3d,g | 1d,g –d,g |
• Neurotoxicityh - Any symptoms - Severe symptoms† | 87d 21d | 52d 2d | 25 3d | 20 –d |
• Nausea and Vomiting - Any symptoms - Severe symptoms† | 88 18 | 93 24 | 65 10 | 69 11 |
• Myalgia/Arthralgia - Any symptoms - Severe symptoms† | 60d 6 d | 27d 1d | 9d 1 | 2d – |
• Diarrhea - Any symptoms - Severe symptoms† | 37d 2 | 29d 3 | 16d 4 | 8d 1 |
• Asthenia - Any symptoms - Severe symptoms† | NC NC | NC NC | 17d 1 | 10d 1 |
• Alopecia - Any symptoms - Severe symptoms† | 96d 51d | 89d 21d | 55d 6 | 37d 8 |
| a Based on worst course analysis. b Paclitaxel (T) dose in mg/m2/infusion duration in hours. c Cyclophosphamide (C) or cisplatin (c) dose in mg/m2. d p<0.05 by Fisher exact test. e <130,000/mm3 in the Intergroup study. f <12 g/dL in the Intergroup study. g All patients received premedication. h In the GOG-111 study, neurotoxicity was collected as peripheral neuropathy and in the Intergroup study, neuro toxicity was collected as either neuromotor or neurosensory symptoms. † Severe events are defined as at least Grade III toxicity. NC Not Collected | ||||
| | Percent of Patients | |||
175/3b (n=95) | 175/24b (n=105) | 135/3b (n=98 ) | 135/24b (n=105) | |
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Thrombocytopenia <100,000/mm3 <50,000/mm3 - Anemia <11 g/dL <8 g/dL - Infections | 78 27 4 1 84 11 26 | 98 75 18 7 90 12 29 | 78 14 8 2 68 6 20 | 98 67 6 1 88 10 18 |
• Hypersensitivity Reactionc - All - Severe† | 41 2 | 45 0 | 38 2 | 45 1 |
• Peripheral Neuropathy - Any symptoms - Severe symptoms† | 63 1 | 60 2 | 55 0 | 42 0 |
• Mucositis - Any symptoms - Severe symptoms† | 17 0 | 35 3 | 21 0 | 25 2 |
| a Based on worst course analysis. b Paclitaxel dose in mg/m2/infusion duration in hours c All patients received premedication. † Severe events are defined as at least Grade III toxicity. | ||||
Myelosuppression was dose and schedule related, with the schedule effect being more prominent. The development of severe hypersensitivity reactions (HSRs) was rare; 1% of the patients and 0.2% of the courses overall. There was no apparent dose or schedule effect seen for the HSRs. Peripheral neuropathy was clearly dose-related, but schedule did not appear to affect the incidence.
| | Percent of Patients | |||
Early Population | Total Population | |||
ACc (n=166) | ACc followed by Td (n=159 ) | ACc (n=1551) | ACc followed by Td (n=1570 ) | |
• Bone Marrowe - Neutropenia <500/mm3 - Thrombocytopenia <50,000/mm3 - Anemia <8 g/dL - Infections - Fever Without Infection | 79 27 17 6 – | 76 25 21 14 3 | 48 11 8 5 <1 | 50 11 8 6 1 |
• Hypersensitivity Reactionf | 1 | 4 | 1 | 2 |
• Cardio vascular Events | 1 | 2 | 1 | 2 |
• Neuromotor Toxicity | 1 | 1 | <1 | 1 |
• Neurosensory Toxicity | – | 3 | <1 | 3 |
• Myalga/Arthralgia | – | 2 | <1 | 2 |
• Nausea/Vomiting | 13 | 18 | 8 | 9 |
• Mucositis | 13 | 4 | 6 | 5 |
| a Based on worst course analysis. b Severe events are defined as at least Grade III toxicity. c Patients received 600 mg /m2 cyclophosphamide and doxorubicin (AC) at doses of either 60 mg/m2, 75 mg/m2, or 90 mg/m2 (with prophylactic G-CSF support and ciprofloxacin), every 3 weeks for 4 courses. d Paclitaxel (T) following 4 courses of AC at a dose of 175 mg/m2/3 hours every 3 weeks for 4 courses. e The incidence of febrile neutropenia was not reported in this study. f All patients were to receive premedication. | ||||
The incidence of an adverse event for the total population likely represents an underestimation of the actual incidence given that safety data were collected differently based on enrollment cohort. However, since safety data were collected consistently across regimens, the safety of the sequential addition of paclitaxel following AC therapy may be compared with AC therapy alone. Compared to patients who received AC alone, patients who received AC followed by paclitaxel experienced more Grade III/IV neurosensory toxicity, more Grade III/IV myalgia/arthralgia, more Grade III/IV neurologic pain (5% vs 1%), more Grade III/IV flu-like symptoms (5% vs 3%), and more Grade III/IV hyperglycemia (3% vs 1%). During the additional 4 courses of treatment with paclitaxel, 2 deaths (0.1%) were attributed to treatment. During paclitaxel treatment, Grade IV neutropenia was reported for 15% of patients, Grade II/III neurosensory toxicity for 15%, Grade II/III myalgias for 23%, and alopecia for 46%.
The incidences of severe hematologic toxicities, infections, mucositis, and cardio vascular events increased with higher doses of doxorubicin.
| | Percent of patients | |
175/3b (n=229) | 135/3b (n=229) | |
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Thrombocytopenia <100,000/mm3 <50,000/mm3 - Anemia <11 g/dL <8 g/dL - Infections - Febrile Neutropenia | 90 28 11 3 55 4 23 2 | 81 19 7 2 47 2 15 2 |
• Hypersensitivity Reactionc - All - Severe† | 36 0 | 31 <1 |
• Peripheral Neuropathy - Any symptoms - Severe symptoms† | 70 7 | 46 3 |
• Mucositis - Any symptoms - Severe symptoms† | 23 3 | 17 <1 |
| a Based on worst course analysis. b Paclitaxel dose in mg/m2/infusion duration in hours. c All patients received premedication. † Severe events are defined as at least Grade III Toxicity. | ||
Myelosuppression and peripheral neuropathy were dose related. There was one severe hypersensitivity reaction (HSR) observed at the dose of 135 mg/m2.
The following table shows the incidence of important adverse events.
| | Percent of Patients | ||
|---|---|---|---|
T135/24b c75 (n=195) | T250/24c c75 (n=197) | VP100 d c75 (n=196) | |
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Thrombocytopenia <50,000/mm3 - Anemia <8 g/dL - Infections | 89 74e 48 6 94 22 38 | 86 65 68 12 96 19 31 | 84 55 62 16 95 28 35 |
• Hypersensitivity Reactionf - All - Severe† | 16 1 | 27 4e | 13 1 |
• Arthralgia/Myalgia - Any symptoms - Severe symptoms† | 21e 3 | 42e 11 | 9 1 |
• Nausea/Vomiting - Any symptoms - Severe symptoms† | 85 27 | 87 29 | 81 22 |
| • Mucositis - Any symptoms - Severe symptoms† | 18 1 | 28 4 | 16 2 |
• Neuromotor Toxicity - Any symptoms - Severe symptoms† | 37 6 | 47 12 | 44 7 |
• Neurosensory Toxicity - Any symptoms - Severe symptoms† | 48 13 | 61 28e | 25 8 |
• Cardiovascular Events - Any symptoms - Severe symptoms† | 33 13 | 39 12 | 24 8 |
| a Based on worst course analysis. b Paclitaxel (T) dose in mg/m2/infusion duration in hours; cisplatin (c) dose in mg/m2. c Paclitaxel dose in mg/m2/infusion duration in hours with G-CSF support; cisplatin dose in mg /m2. d Etoposide (VP) dose in mg/m2 was administered IV on days 1, 2, and 3; cisplatin dose in mg /m2. e p<0.05. f All patients received premedication. † Severe events are defined as at least Grade III Toxicity. | |||
Toxicity was generally more severe in the high-dose paclitaxel treatment arm (T250/c75) than in the low-dose paclitaxel arm (T135/c75). Compared to the cisplatin/etoposide arm, patients in the low-dose paclitaxel arm experienced more arthralgia/myalgia of any grade and more severe neutropenia. The incidence of febrile neutropenia was not reported in this study.
| | Percent of Patients | |
Study CA139-174 Paclitaxel 135/3b q 3 wk (n=29) | Study CA139-281 Paclitaxel 100/3b q 2 wk (n=56) | |
• Bone Marrow - Neutropenia <2,000/mm3 <500/mm3 - Thrombocytopenia <100,000/mm3 <50,000/mm3 - Anemia <11 g/dL <8 g/dL - Febrile Neutropenia | 100 76 52 17 86 34 55 | 95 35 27 5 73 25 9 |
• Opportunistic Infection - Any - Cytomegalovirus - Herpes Simplex - Pneumocystis carinii - M. avinum intracellulare - Candidiasis, esophageal - Cryptosporidiosis - Cryptococcal meningitis - Leukoencephalopathy | 76 45 38 14 24 7 7 3 - | 54 27 11 21 4 9 7 2 2 |
• Hypersensitivity Reactionc - All | 14 | 9 |
• Cardio vascular - Hypotension - Bradycardia | 17 3 | 9 – |
| • Peripheral Neuropathy - Any - Severe† | 79 10 | 46 2 |
• Myalgia/Arthralgia - Any - Severe† | 93 14 | 48 16 |
• Gastrointestinal - Nausea and Vomiting - Diarrhea - Mucositis | 69 90 45 | 70 73 20 |
• Renal (creatinine elevation) - Any - Severe† | 34 7 | 18 5 |
Discontinuation for drug toxicity | 7 | 16 |
| a Based on worst course analysis. b Paclitaxel dose in mg/m2/infusion duration in hours. c All patients received premedication. † Severe events are defined as at least Grade III toxicity. | ||
As demonstrated in this table, toxicity was more pronounced in the study utilizing paclitaxel at a dose of 135 mg/m2 every 3 weeks than in the study utilizing palcitaxel at a dose of 100 mg/m2 every 2 weeks. Notably, severe neutropenia (76% vs 35%), febrile neutropenia (55% vs 9%), and opportunistic infections (76% vs 54%) were more common with the former dose and schedule. The differences between the 2 studies with respect to dose escalation and use of hematopoietic growth factors, as described above, should be taken into account. (See
The following discussion refers to the overall safety database of 812 patients with solid tumors treated with single-agent paclitaxel in clinical studies. Toxicities that occurred with greater severity or frequency in previously untreated patients with ovarian carcinoma or NSCLC who received paclitaxel in combination with cisplatin or in patients with breast cancer who received paclitaxel after doxorubicin/cyclophosphamide in the adjuvant setting and that occurred with a difference that was clinically significant in these populations are also described.
The frequency and severity of important adverse events for the Phase 3 ovarian carcinoma, breast carcinoma, NSCLC, and the Phase 2 Kaposi’s sarcoma carcinoma studies are presented bove in tabular form by treatment arm. In addition, rare events have been reported from postmarketing experience or from other clinical studies. The frequency and severity of adverse events have been generally similar for patients receiving paclitaxel for the treatment of ovarian, breast, or lung carcinoma or Kaposi’s sarcoma, but patients with AIDS-related Kaposi’s sarcoma may have more frequent and severe hematologic toxicity, infections (including opportunistic infections, see Table 16), and febrile neutropenia. These patients require a lower dose intensity and supportive care. (See
In the study where paclitaxel was administered to patients with ovarian carcinoma at a dose of 135 mg/m2/24 hours in combination with cisplatin versus the control arm of cyclophosphamide plus cisplatin, the incidences of grade IV neutropenia and of febrile neutropenia were significantly greater in the paclitaxel plus cisplatin arm than in the control arm. Grade IV neutro penia occurred in 81% on the paclitaxel plus cisplatin arm versus 58% on the cyclophosphamide plus cisplatin arm, and febrile neutropenia occurred in 15% and 4% respectively. On the paclitaxel/cisplatin arm, there were 35/1074 (3%) courses with fever in which Grade IV neutro penia was reported at some time during the course. When paclitaxel followed by cisplatin was administered to patients with advanced NSCLC in the ECOG study, the incidences of Grade IV neutropenia were 74% (paclitaxel 135 mg/m2/24 hours followed by cisplatin) and 65% (paclitaxel 250 mg/m2/24 hours followed by cisplatin and G-CSF) compared with 55% in patients who received cisplatin/etoposide.
Fever was frequent (12% of all treatment courses). Infectious episodes occurred in 30% of all patients and 9% of all courses; these episodes were fatal in 1% of all patients, and included sepsis, pneumonia and peritonitis. In the Phase 3 second-line ovarian study, infectious episodes were reported in 20% and 26% of the patients treated with a dose of 135 mg/m2 or 175 mg/m2 given as a 3-hour infusion respectively. Urinary tract infections and upper respiratory tract infections were the most frequently reported infectious complications. In the immunosuppressed patient population with advanced HIV disease and poor-risk AIDS-related Kaposi’s sarcoma, 61% of the patients reported at least one opportunistic infection. (See
Thrombocytopenia was reported. Twenty percent of the patients experienced a drop in their platelet count below 100,000 cells/mm3 at least once while on treatment; 7% had a platelet count <50,000 cells/mm3 at the time of their wo rst nadir. Bleeding episodes were reported in 4 of all courses and by 14% of all patients but most of the hemorrhagic episodes were localized and the frequency of these events was unrelated to the Paclitaxel Injection, USP dose and schedule. In the Phase 3 second-line ovarian study, bleeding episodes were reported in 10% of the patients; no patients treated with the 3-hour infusion received platelet transfusions. In the adjuvant breast carcinoma trial, the incidence of severe thrombocytopenia and platelet transfusions increased with higher doses of doxorubicin.
Anemia (Hb <11 g/dL) was observed in 78% o f all patients and was severe (Hb <8 g/dL) in 16% of the cases. No consistent relationship between dose or schedule and the frequency of anemia was observed. Among all patients with normal baseline hemoglobin, 69% became anemic on study but only 7% had severe anemia. Red cell transfusions were required in 25% o f all patients and in 12% of those with normal baseline hemoglobin levels.
The minor hypersensitivity reactions consisted mostly of flushing (28%), rash (12%), hypotension (4%), dyspnea (2%), tachycardia (2%), and hypertension (1%). The frequency of hypersensitivity reactions remained relatively stable during the entire treatment period.
Chills, shock, and back pain in association with hypersensitivity reactions have been reported.
Significant cardiovascular events possibly related to single-agent paclitaxel occurred in approximately 1% of all patients. These events included syncope, rhythm abnormalities, hypertension and venous thrombosis. One of the patients with syncope treated with paclitaxel at 175 mg/m2 over 24 hours had progressive hypotension and died. The arrhythmias included asymptomatic ventricular tachycardia, bigeminy and complete AV block requiring pacemaker placement. Among patients with NSCLC treated with paclitaxel in combination with cisplatin in the Phase 3 study, significant cardiovascular events occurred in 12 to 13%. This apparent increase in cardiovascular events is possibly due to an increase in cardiovascular risk factors in patients with lung cancer.
Electrocardiogram (ECG) abnormalities were common among patients at baseline. ECG abnormalities on study did not usually result in symptoms, were not dose-limiting, and required no intervention. ECG abnormalities were noted in 23% of all patients. Among patients with a normal ECG prior to study entry, 14% of all patients developed an abnormal tracing while on study. The most frequently reported ECG modifications were non-specific repolarization abnormalities, sinus bradycardia, sinus tachycardia, and premature beats. Among patients with normal ECGs at baseline, prior therapy with anthracyclines did not influence the frequency of ECG abnormalities.
Cases of myocardial infarction have been reported. Congestive heart failure, including cardiac dysfunctio n and reduction of left ventricular ejection fraction or ventricular failure, has been reported typically in patients who have received o ther chemo therapy, notably anthracyclines. (See
Atrial fibrillation and supraventricular tachycardia have been reported.
Pleural effusion and respiratory failure have been reported.
In general, the frequency and severity of neurologic manifestations were dose-dependent in patients receiving single-agency paclitaxel. Peripheral neuropathy was observed in 60% of all patients (3% severe) and in 52% (2% severe) of the patients without pre-existing neuropathy. The frequency of peripheral neuropathy increased with cumulative dose. Paresthesia commonly occurs in the form of hyperesthesia. Neurologic symptoms were observed in 27% of the patients after the first course of treatment and in 34% to 51% from course2 to 10. Peripheral neuropathy was the cause of paclitaxel discontinuation in 1% of all patients. Sensory symptoms have usually improved or resolved within several months of paclitaxel discontinuation. Pre-existing neuropathies resulting from prior therapies are not a contraindication for paclitaxel therapy.
In the Intergroup first-line ovarian carcinoma study (see Table 11), neurotoxicity included reports of neuromotor and neurosensory events. The regimen with paclitaxel 175 mg/m2 given by 3-hour infusion plus cisplatin 75 mg/m2 resulted in greater incidence and severity of neurotoxicity than the regimen containing cyclophosphamide and cisplatin, 87% (21% severe) versus 52% (2% severe), respectively. The duration of grade III or IV neurotoxicity cannot be determined with precision for the Intergroup study since the resolution dates of adverse events were not collected in the case report forms for this trial and complete follow-up documentation was available only in a minority of these patients. In the GOG first-line ovarian carcinoma study, neurotoxicity was reported as peripheral neuropathy. The regimen with paclitaxel 135 mg/m2 given by 24-hour infusion plus cisplatin 75 mg/m2 resulted in an incidence of neurotoxicity that was similar to the regimen containing cyclophosphamide plus cisplatin, 25% (3% severe) versus 20% (0% severe), respectively. Cross-study comparison of neurotoxicity in the Intergroup and GOG trials suggests that when paclitaxel is given in combination with cisplatin 75 mg/m2, the incidence of severe neurotoxicity is more common at a paclitaxel dose of 175 mg/m2 given by 3-hour infusion (21%) than at a dose of 135 mg/m2 given by 24-hour infusion (3%).
In patients with NSCLC, administration of paclitaxel followed by cisplatin resulted in a greater incidence of severe neurotoxicity compared to the incidence in patients with ovarian or breast cancer treated with single-agent paclitaxel. Severe neurosensory symptoms were noted in 13% of NSCLC patients receiving paclitaxel 135 mg/m2 by 24-hour infusion followed by cisplatin 75 mg/m2 and 8% of NSCLC patients receiving cisplatin/etoposide (see Table 15).
Other than peripheral neuropathy, serious neurologic events following paclitaxel administration have been rare (<1%) and have included grand mal seizures, syncope, ataxia, and neuroencephalopathy.
Autonomic neuropathy resulting in paralytic ileus have been reported. Optic nerve and/or visual disturbances (scintillating scotomata) have also been reported, particularly in patients who have received higher doses than those recommended. These effects generally have been reversible. However, rare reports in the literature of abnormal visual evoked potentials in patients have suggested persistent optic nerve damage. Postmarketing reports of ototoxicity (hearing loss and tinnitus) have also been received.
Convulsions, dizziness, and headache have been reported.
Hepatic:
Hepatic necrosis and hepatic encephalopathy leading to death have reported.
Patients with gynecological cancers treated with paclitaxel and cisplatin may have an increased risk of renal failure with the combination therapy of paclitaxel and cisplatin in gynecological cancers as compared to cisplatin alone.
In patients with poor-risk AIDS-related Kaposi’s sarcoma, nausea/vomiting, diarrhea, and mucositis were reported by 69%, 79%, and 28% of patients, respectively. One-third of 43 patients with Kaposi’s sarcoma complained of diarrhea prior to study start. (See
In the first-line Phase 3 ovarian carcinoma studies, the incidence of nausea and vomiting when paclitaxel was administered in combination with cisplatin appeared to be greater compared with the database for single-agent paclitaxel in ovarian and breast carcinoma. In addition, diarrhea of any grade was reported more frequently compared to the control arm, but there was no difference for severe diarrhea in these studies.
Intestinal obstruction, intestinal perforation, pancreatitis, ischemic colitis, and dehydration have been reported. Neutropenic enterocolitis (typhlitis), despite the coadministration of G-CSF, were observed in patients treated with paclitaxel alone and in combination with other chemotherapeutic agents.
More severe events such as phlebitis, cellulitis, induration, skin exfoliation, necrosis, and fibrosis have been reported. In some cases the onset of the injection site reaction either occurred during a prolonged infusion or was delayed by a week to ten days.
A specific treatment for extravasation reactions is unknown at this time. Given the possibility of
extravasation, it is advisable to closely monitor the infusion site for possible infiltration during drug administration.
Skin abnormalities related to radiation recall as well as reports of maculopapular rash, pruritus, Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported. In postmarketing experience, diffuse edema, thickening, and sclerosing of the skin have been reported following paclitaxel administration. Paclitaxel has been reported to exacerbate signs and symptoms of scleroderma.
Reports of asthenia and malaise have been received as part of the continuing surveillance of paclitaxel safety. In the Phase 3 trial of paclitaxel 135 mg/m over 24 hours in combination with cisplatin as firstline therapy of ovarian cancer, asthenia was reported in 17% of the patients, significantly greater than the 10% incidence observed in the control arm of cyclophosphamide/cisplatin.
Conjunctivitis, increased lacrimation, anorexia, confusional state, photopsia, visual floaters, vertigo, and increase in blood creatinine have been reported.
Paclitaxel Injection, USP is a clear colorless to slightly yellow viscous solution. It is supplied as a nonaqueous solution intended for dilution with a suitable parenteral fluid prior to intravenous infusion. Paclitaxel is available in 30 mg (5 mL), 100 mg (16.7 mL), and 300 mg (50 mL) multidose vials. Each mL of sterile nonpyrogenic solution contains 6 mg paclitaxel, 527 mg of Polyoxyl 35 Castor Oil, NF, 49.7% (v/v) Dehydrated Alcohol, USP and 2 mg Citric Acid, USP.
Paclitaxel is a natural product with antitumor activity. Paclitaxel is obtained via an extraction process from
Paclitaxel has the following structural formula:
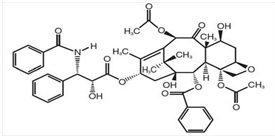
Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and molecular weight of 853.9. It is highly lipophilic, insoluble in water, and melts at around 216 to 217°C.