Pemetrexed
Pemetrexed Prescribing Information
Indications and Usage | 11/2022 |
Indications and Usage | 11/2022 |
Dosage and Administration | 11/2022 |
Warnings and precautions | 11/2022 |
Injection: Pemetrexed Injection is a clear, colorless to slightly yellowish or slightly yellow-greenish solution available in sterile single-dose vials containing 100 mg/4 mL, 500 mg/20 mL, and 1 g/40 mL of pemetrexed.
Pemetrexed is contraindicated in patients with a history of severe hypersensitivity reaction to pemetrexed
The following serious adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions ]
- Renal failure [see Warnings and Precautions ]
- Bullous and exfoliative skin toxicity [see Warning and Precautions ]
- Interstitial pneumonitis [see Warnings and Precautions ]
- Radiation recall [see Warnings and Precautions ]
Ibuprofen increases exposure (AUC) of pemetrexed
- Avoid administration of ibuprofen for 2 days before, the day of, and 2 days following administration of Pemetrexed Injection [see Dosage and Administration ].
- Monitor patients more frequently for myelosuppression, renal, and gastrointestinal toxicity, if concomitant administration of ibuprofen cannot be avoided.
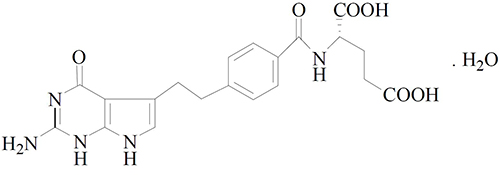
Pemetrexed is a folate analog metabolic inhibitor. The drug substance, pemetrexed diacid monohydrate, has the chemical name N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1
Pemetrexed Injection is supplied as a sterile solution for intravenous infusion available in single-dose vials. The product is a clear to slightly yellowish or slightly yellow-greenish solution. Each mL contains 25 mg pemetrexed, 35 mg tromethamine, 10 mg citric acid anhydrous, 0.5 mg methionine and water for injection. Tromethamine may have been added to adjust pH.