Penicillamine - Penicillamine tablet, Film Coated
(Penicillamine)Penicillamine - Penicillamine tablet, Film Coated Prescribing Information
Penicillamine tablets are indicated in the treatment of Wilson’s disease, cystinuria, and in patients with severe, active rheumatoid arthritis who have failed to respond to an adequate trial of conventional therapy. Available evidence suggests that penicillamine tablets are not of value in ankylosing spondylitis.
Two types of patients require treatment for Wilson’s disease: (1) the symptomatic, and (2) the asymptomatic in whom it can be assumed the disease will develop in the future if the patient is not treated.
Diagnosis, suspected on the basis of family or individual history, physical examination, or a low serum concentration of ceruloplasmin*, is confirmed by the demonstration of Kayser-Fleischer rings or, particularly in the asymptomatic patient, by the quantitative demonstration in a liver biopsy specimen of a concentration of copper in excess of 250 mcg/g dry weight.
Treatment has two objectives:
(1) to minimize dietary intake and absorption of copper.
(2) to promote excretion of copper deposited in tissues.
The first objective is attained by a daily diet that contains no more than one or two milligrams of copper. Such a diet should exclude, most importantly, chocolate, nuts, shellfish, mushrooms, liver, molasses, broccoli, and cereals enriched with copper, and be composed to as great an extent as possible of foods with a low copper content. Distilled or demineralized water should be used if the patient’s drinking water contains more than 0.1 mg of copper per liter.
For the second objective, a copper chelating agent is used.
In symptomatic patients, this treatment usually produces marked neurologic improvement, fading of Kayser-Fleischer rings, and gradual amelioration of hepatic dysfunction and psychic disturbances.
Clinical experience to date suggests that life is prolonged with the above regimen.
Noticeable improvement may not occur for one to three months. Occasionally, neurologic symptoms become worse during initiation of therapy with penicillamine tablets. Despite this, the drug should not be discontinued permanently. Although temporary interruption may result in clinical improvement of the neurological symptoms, it carries an increased risk of developing a sensitivity reaction upon resumption of therapy (See
* For quantitative test for serum ceruloplasmin see: Morell, A.G.; Windsor, J.; Sternlieb, I; Scheinberg, I.H.: Measurement of the concentration of ceruloplasmin in serum by determination of its oxidase activity, in “Laboratory Diagnosis of Liver Disease,” F.W. Sunderman; F.W. Sunderman, Jr., (eds.), St. Louis, Warren H. Green, Inc., 1968, pp. 193-195.
Treatment of asymptomatic patients has been carried out for over ten years. Symptoms and signs of the disease appear to be prevented indefinitely if daily treatment with penicillamine tablets can be continued.
Arginine, lysine, ornithine, and cysteine are soluble substances, readily excreted. There is no apparent pathology connected with their excretion in excessive quantities.
Cystine, however, is so slightly soluble at the usual range of urinary pH that it is not excreted readily, and so crystallizes and forms stones in the urinary tract. Stone formation is the only known pathology in cystinuria. Normal daily output of cystine is 40 to 80 mg. In cystinuria, output is greatly increased and may exceed 1 g/day. At 500 to 600 mg/day, stone formation is almost certain. When it is more than 300 mg/day, treatment is indicated.
Conventional treatment is directed at keeping urinary cystine diluted enough to prevent stone formation, keeping the urine alkaline enough to dissolve as much cystine as possible, and minimizing cystine production by a diet low in methionine (the major dietary precursor of cystine). Patients must drink enough fluid to keep urine specific gravity below 1.010, take enough alkali to keep urinary pH at 7.5 to 8, and maintain a diet low in methionine. This diet is not recommended in growing children and probably is contraindicated in pregnancy because of its low protein content (see
When these measures are inadequate to control recurrent stone formation, penicillamine tablets may be used as additional therapy. When patients refuse to adhere to conventional treatment, penicillamine tablets may be a useful substitute. It is capable of keeping cystine excretion to near normal values, thereby hindering stone formation and the serious consequences of pyelonephritis and impaired renal function that develop in some patients.
Bartter and colleagues depict the process by which penicillamine interacts with cystine to form penicillamine-cysteine mixed disulfide as:
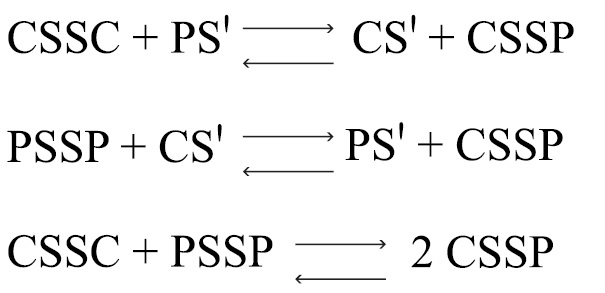
CSSC = cystine
CS' = deprotonated cysteine
PSSP = penicillamine
PS' = deprotonated penicillamine sulfhydryl
CSSP = penicillamine-cysteine mixed disulphide
In this process, it is assumed that the deprotonated form of penicillamine, PS', is the active factor in bringing about the disulfide interchange.
In all patients receiving penicillamine, it is important that penicillamine tablets be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food, or milk. Because penicillamine increases the requirement for pyridoxine, patients may require a daily supplement of pyridoxine (see
Determination of 24-hour urinary copper excretions is of greatest value in the first week of therapy with penicillamine. In the absence of any drug reaction, a dose between 0.75 and 1.5 g that results in an initial 24-hour cupriuresis of over 2 mg should be continued for about three months, by which time the most reliable method of monitoring maintenance treatment is the determination of free copper in the serum. This equals the difference between quantitatively determined total copper and ceruloplasmin-copper. Adequately treated patients will usually have less than 10 mcg free copper/dL of serum. It is seldom necessary to exceed a dosage of 2 g/day. If the patient is intolerant to therapy with penicillamine tablets, alternative treatment is trientine hydrochloride.
In patients who cannot tolerate as much as 1 g/day initially, initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions.
The usual dosage of penicillamine tablets in the treatment of cystinuria is 2 g/day for adults, with a range of 1 to 4 g/day. For pediatric patients, dosage can be based on 30 mg/kg/day. The total daily amount should be divided into four doses. If four equal doses are not feasible, give the larger portion at bedtime. If adverse reactions necessitate a reduction in dosage, it is important to retain the bedtime dose.
Initiating dosage with 250 mg/day, and increasing gradually to the requisite amount, gives closer control of the effects of the drug and may help to reduce the incidence of adverse reactions.
In addition to taking penicillamine tablets, patients should drink copiously. It is especially important to drink about a pint of fluid at bedtime and another pint once during the night when urine is more concentrated and more acid than during the day. The greater the fluid intake, the lower the required dosage of penicillamine tablets.
Dosage must be individualized to an amount that limits cystine excretion to 100 to 200 mg/day in those with no history of stones, and below 100 mg/day in those who have had stone formation and/or pain. Thus, in determining dosage, the inherent tubular defect, the patient’s size, age, and rate of growth, and his diet and water intake all must be taken into consideration.
The standard nitroprusside cyanide test has been reported useful as a qualitative measure of the effective dose*:
* Lotz, M., Potts, J.T. and Bartter, F.C.:
Add 2 mL of freshly prepared 5 percent sodium cyanide to 5 mL of a 24-hour aliquot of protein-free urine and let stand ten minutes. Add 5 drops of freshly prepared 5 percent sodium nitroprusside and mix. Cystine will turn the mixture magenta. If the result is negative, it can be assumed that cystine excretion is less than 100 mg/g creatinine.
Although penicillamine is rarely excreted unchanged, it also will turn the mixture magenta. If there is any question as to which substance is causing the reaction, a ferric chloride test can be done to eliminate doubt: Add 3 percent ferric chloride dropwise to the urine. Penicillamine will turn the urine an immediate and quickly fading blue. Cystine will not produce any change in appearance.
When treatment with penicillamine tablets has been interrupted because of adverse reactions or other reasons, the drug should be reintroduced cautiously by starting with a lower dosage and increasing slowly.
Except for treatment of Wilson’s disease or certain cases of cystinuria, use of penicillamine during pregnancy is contraindicated (See
Although breast milk studies have not been reported in animals or humans, mothers on therapy with penicillamine should not nurse their infants.
Patients with a history of penicillamine-related aplastic anemia or agranulocytosis should not be restarted on penicillamine (see
Penicillamine is a drug with a high incidence of untoward reactions, some of which are potentially fatal. Therefore, it is mandatory that patients receiving penicillamine therapy remain under close medical supervision throughout the period of drug administration (see
Reported incidences (%) for the most commonly occurring adverse reactions in rheumatoid arthritis patients are noted, based on 17 representative clinical trials reported in the literature (1270 patients).
Urticaria and exfoliative dermatitis have occurred.
Thyroiditis has been reported; hypoglycemia in association with anti-insulin antibodies has been reported. These reactions are extremely rare.
Some patients may develop a migratory polyarthralgia, often with objective synovitis (see
Isolated cases of reactivated peptic ulcer have occurred, as have hepatic dysfunction and pancreatitis. Intrahepatic cholestasis and toxic hepatitis have been reported rarely. There have been a few reports of increased serum alkaline phosphatase, lactic dehydrogenase, and positive cephalin flocculation and thymol turbidity tests.
Some patients may report a blunting, diminution, or total loss of taste perception (12%); or may develop oral ulcerations. Although rare, cheilosis, glossitis, and gingivostomatitis have been reported (see
Gastrointestinal side effects are usually reversible following cessation of therapy.
Thrombotic thrombocytopenic purpura, hemolytic anemia, red cell aplasia, monocytosis, leukocytosis, eosinophilia, and thrombocytosis have also been reported.
Increased skin friability, excessive wrinkling of skin, and development of small, white papules at venipuncture and surgical sites have been reported (see
The chelating action of the drug may cause increased excretion of other heavy metals such as zinc, mercury, and lead.
There have been reports associating penicillamine with leukemia. However, circumstances involved in these reports are such that a cause and effect relationship to the drug has not been established.
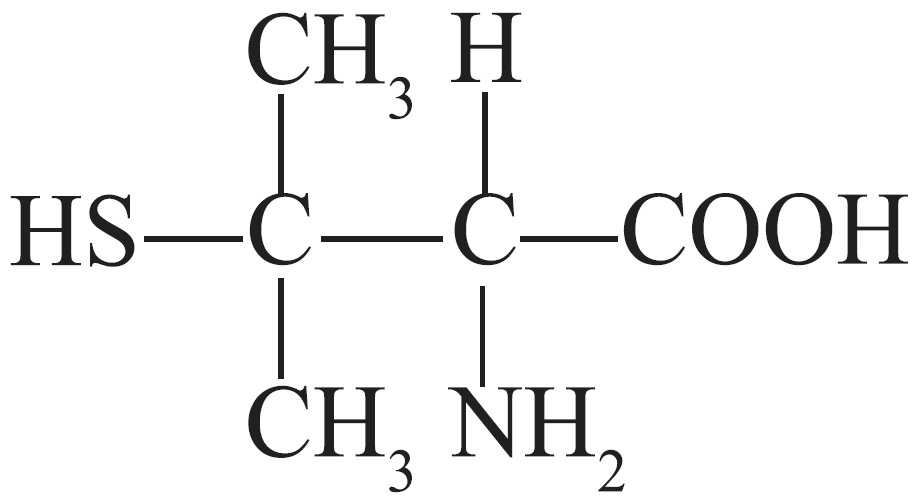
Penicillamine is 3-mercapto-D-valine, a disease modifying antirheumatic drug. It is a white or practically white, crystalline powder, freely soluble in water, slightly soluble in alcohol, and insoluble in ether, acetone, benzene, and carbon tetrachloride. Although its configuration is D, it is levorotatory as usually measured:
[α]25° = -62.5° ± 2.0° (C = 1, 1
D
The empirical formula is C5H11NO2S, giving it a molecular weight of 149.21. The structural formula is:
It reacts readily with formaldehyde or acetone to form a thiazolidine-carboxylic acid.
Penicillamine tablets, USP (Titratable Tablets) for oral administration contain 250 mg of penicillamine.
Other ingredients (inactive): anhydrous lactose, copovidone, corn starch (maize), edetate disodium dihydrate, hypromellose, lactose monohydrate, magnesium stearate, magnesium trisilicate, polyethylene glycol, povidone and stearic acid.
Penicillamine is a chelating agent recommended for the removal of excess copper in patients with Wilson’s disease. From
Penicillamine also reduces excess cystine excretion in cystinuria. This is done, at least in part, by disulfide interchange between penicillamine and cystine, resulting in formation of penicillamine-cysteine disulfide, a substance that is much more soluble than cystine and is excreted readily.
Penicillamine interferes with the formation of cross-links between tropocollagen molecules and cleaves them when newly formed.
The mechanism of action of penicillamine in rheumatoid arthritis is unknown, although it appears to suppress disease activity. Unlike cytotoxic immunosuppressants, penicillamine markedly lowers IgM rheumatoid factor but produces no significant depression in absolute levels of serum immunoglobulins. Also unlike cytotoxic immunosuppressants, which act on both, penicillamine
In rheumatoid arthritis, the onset of therapeutic response to penicillamine may not be seen for two or three months. In those patients who respond, however, the first evidence of suppression of symptoms such as pain, tenderness, and swelling usually is generally apparent within three months. The optimum duration of therapy has not been determined. If remissions occur, they may last from months to years but usually require continued treatment (see
In all patients receiving penicillamine, it is important that penicillamine be given on an empty stomach, at least one hour before meals or two hours after meals, and at least one hour apart from any other drug, food or milk. This permits maximum absorption and reduces the likelihood of inactivation by metal binding in the gastrointestinal tract.
Methodology for determining the bioavailability of penicillamine is not available; however, penicillamine is known to be a very soluble substance.