Penicillin G Potassium - Penicillin G Potassium injection, Powder, For Solution
(Penicillin G Potassium)Penicillin G Potassium - Penicillin G Potassium injection, Powder, For Solution Prescribing Information
Buffered penicillin G potassium for injection is indicated in the treatment of serious infections caused by susceptible strains of the designated microorganisms in the conditions listed below. Appropriate culture and susceptibility tests should be done before treatment in order to isolate and identify organisms causing infection and to determine their susceptibility to penicillin G. Therapy with Buffered penicillin G potassium for injection may be initiated before results of such tests are known when there is reason to believe the infection may involve any of the organisms listed below, however, once these results become available, appropriate therapy should be continued.
CLINICAL INDICATION | INFECTING ORGANISM |
|---|---|
Septicemia, empyema, pneumonia, pericarditis, endocarditis, meningitis | Streptococcus pyogenes (group A beta-hemolytic streptococcus ), other beta-hemolytic streptococci including groups C, H, G, L and M, Streptococcus pneumoniae and Staphylococcus species (non-penicillinase producing strains) |
Anthrax | Bacillus anthracis |
Actinomycosis (cervicofacial disease and thoracic and abdominal disease) | Actinomyces Israelil |
Botulism (adjunctive therapy to antitoxin), gas gangrene, and tetanus (adjunctive therapy to human tetanus immune globulin) | Clostridium species |
Diphtheria (adjunctive therapy to antitoxin and prevention of the carrier state) | Corynebacterium diphtheriae |
Erysipelothrix endocarditis | Erysipelothrix rhusiopthiae |
Fusospirochetosis (severe infections of the oropharynx [Vincent’s], lower respiratory tract and genital area) | Fusobacterium species and spirochetes |
Listeria infections including meningitis and endocarditis | Listeria monocytogenes |
Pasteurella infections including bacteremia and meningitis | Pasteurella multocida |
Haverhill fever | Streptobacillus moniliformis |
Rat-bite fever | Spirillum minus or Streptobacillus moniliformis |
Disseminated gonococcal infections | Neisseria gonorrhoeae (penicillin-susceptible) |
Syphilis (congenital and neurosyphilis) | Treponema pallidum |
Meningococcal meningitis and/or septicemia | Neisseria meningitidis |
Gram-negative bacillary infections (bacteremias) | Escherichia coli, Enterobacter aerogenes, Alcaligenes faecalis, salmonella, shigella and Proteus mirabilis, Penicillin G is not the drug of choice in the treatment of gram-negative bacillary infections. |
To reduce the development of drug-resistant bacteria and maintain the effectiveness of penicillin G potassium and other antibacterial drugs, penicillin G potassium should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Buffered penicillin G potassium for injection may be given intravenously or intramuscularly. The usual dose recommendations are as follows:
CLINICAL INDICATION | DOSAGE |
|---|---|
Serious infections due to susceptible strains of streptococci (including S. pneumoniae) and staphylococci-septicemia , empyema, pneumonia, pericarditis, endocarditis and meningitis | 5 to 24 million units/day depending on the infection and its severity administered in equally divided doses every 4 to 6 hours |
Anthrax | Minimum of 8 million units/day in divided doses every 6 hours. Higher doses may be required depending on susceptibility of organism. |
Actinomycosis | |
Cervicofacial disease | 1 to 6 million units/day |
Thoracic and abdominal disease | 10 to 20 million units/day |
Clostridial infections | |
Botulism (adjunctive therapy to antitoxin) | 20 million units/day |
Gas gangrene (debridement and/or surgery as | |
Tetanus (adjunctive therapy to human tetanus | |
Diphtheria (adjunctive therapy to antitoxin and for the prevention of the carrier state) | 2 to 3 million units/day in divided doses for 10 to 12 days |
Erysipelothrix endocarditis | 12 to 20 million units/day for 4 to 6 weeks |
Fusospirochetosis (severe infections of the oropharnyx [Vincent’s], lower respiratory tract and genital area) | 5 to 10 million units/day |
Listeria infections | |
Meningitis | 15 to 20 million units/day for 2 weeks |
Endocarditis | 15 to 20 million units/day for 4 weeks |
Pasteurella infections including bacteremia and meningitis | 4 to 6 million units/day for 2 weeks |
Haverhill fever, Rat-bite fever | 12 to 20 million units/day |
Disseminated gonococcal infections, such as meningitis endocarditis, arthritis, etc., caused by penicillin-susceptible organisms | 10 million units/day; duration depends on the type of infection |
Syphilis (neurosyphilis) | 12 to 24 million units/day, as 2 to 4 MU every 4 hours for 10 to 14 days; many experts recommend additional therapy with Benzathine PCN G 2.4 MU intramuscular weekly for 3 doses after completion of intravenous therapy |
Meningococcal meningitis and/or septicemia | 24 million units/day as 2 million units every 2 hours |
* Because of its short half-life, penicillin G is administered in divided doses, usually every 4 to 6 hours with the exception of meningococcal meningitis/septicemia, i.e., every 2 hours.
A history of hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
The Jarisch-Herxheimer reaction is a systemic reaction, that may occur after the initiation of penicillin therapy in patients with syphilis or other spirochetal infections (i.e., Lyme disease and Relapsing fever). The reaction begins one to two hours after initiation of therapy and disappears within 12 to 24 hours. It is characterized by fever, chills, myalgias, headache, exacerbation of cutaneous lesions, tachycardia, hyperventiliation, vasodilation with flushing and mild hypotension. The pathogenesis of the Herxheimer reaction may be due to the release from the spirochaete of host stable pyrogen.
Bacteriostatic antibacterials (i.e., chloramphenicol, erythromycins, sulfonamides or tetracyclines) may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided. This has been documented
Penicillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins.
Other drugs may compete with penicillin G for renal tubular secretion and thus prolong the serum half-life of penicillin. These drugs include: aspirin, phenylbutazone, sulfonamides, indomethacin, thiazide diuretics, furosemide and ethacrynic acid.
Buffered penicillin G potassium for injection, USP is sterile penicillin G potassium powder for reconstitution. It is an antibacterial agent intended for intravenous or intramuscularly use.
Chemically, penicillin G potassium is monopotassium (2S,5R,6R)-3,3-dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo (3.2.0) heptane-2-carboxylate, and has the following chemical structure:
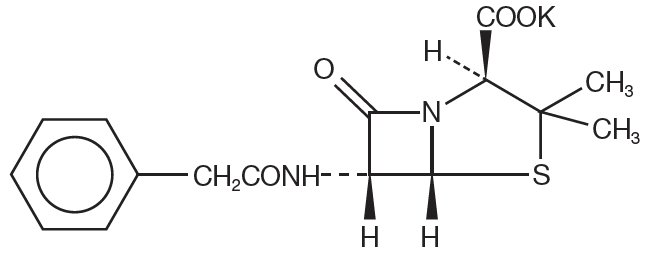
Penicillin G potassium, a water soluble benzylpenicillin, is a white to almost white crystalline powder which is almost odorless and/or after reconstitution a colorless solution. The pH of freshly constituted solutions usually ranges from 6 to 8.5. Sodium citrate and citric acid have been added as a buffer.
Buffered penicillin G potassium for injection, USP is supplied in vials equivalent to 1,000,000 units (1 million units), 5,000,000 units (5 million units), or 20,000,000 units (20 million units) of penicillin G as the potassium salt. Each million unit contains approximately 7.9 milligrams of sodium (0.34 mEq) and 65.6 milligrams of potassium (1.68 mEq).