Phenylephrine Hydrochloride
Phenylephrine Hydrochloride Prescribing Information
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10%, is indicated to dilate the pupil.
Phenylephrine hydrochloride ophthalmic solution, USP 2.5% is a clear, colorless to yellow solution and free of foreign matter, sterile topical ophthalmic solution containing phenylephrine hydrochloride 2.5%: each mL contains 25 mg of phenylephrine hydrochloride.
Phenylephrine hydrochloride ophthalmic solution, USP 10% is a clear, colorless to yellow solution and free of foreign matter, sterile topical ophthalmic solution containing phenylephrine hydrochloride 10%.: each mL contains 100 mg of phenylephrine hydrochloride.
The following serious adverse reactions are described below and elsewhere in the labeling:
- Cardiovascular Effects [See Warnings and Precautions ()]5.2 Cardiovascular Reactions
There have been reports of serious cardiovascular reactions, including ventricular arrhythmias and myocardial infarctions, in patients using phenylephrine 10%. These episodes, some fatal, have usually occurred in patients with pre-existing cardiovascular diseases. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients.
- Elevation in Blood Pressure [See Warnings and Precautions ()]5.3Elevation of Blood Pressure
A significant elevation in blood pressure is not common but has been reported following conjunctival instillation of recommended doses of phenylephrine 10%. The risk is less with phenylephrine 2.5%. Caution should be exercised with the use of phenylephrine 10% in pediatric patients less than 5 years of age and patients with hyperthyroidism, or cardiovascular disease. The post-treatment blood pressure of patients with cardiac and endocrine diseases and any patients who develop symptoms should be carefully monitored.
The following adverse reactions have been identified following use of phenylephrine hydrochloride ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Phenylephrine Hydrochloride Ophthalmic Solution, USP is a sterile, clear, colorless to yellow solution and free of foreign matter, topical α-adrenergic agonist for ophthalmic use. The active ingredient is represented by the chemical structure
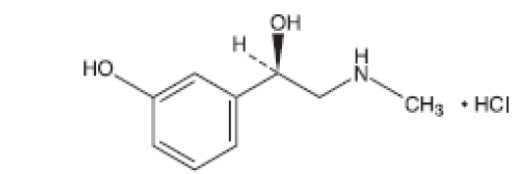
Chemical Name: (R)-3-hydroxy-α[(methylamino)methyl] benzenemethanol hydrochloride.
Molecular Formula: C9H13NO2. HCl
Molecular Weight: 203.67 g/mol
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% contains: ACTIVE: Phenylephrine Hydrochloride 25 mg (2.5%); INACTIVES: Sodium Phosphate Monobasic Anhydrous, Sodium Phosphate Dibasic Anhydrous, Water for Injection. Phosphoric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 to 7.0). The solution has a tonicity of 340 mOsm/kg; PRESERVATIVE: Benzalkonium Chloride 0.1 mg (0.01%).
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% contains: ACTIVE: Phenylephrine Hydrochloride 100 mg (10%); INACTIVES: Sodium Phosphate Monobasic Anhydrous, Sodium Phosphate Dibasic Anhydrous, Water for Injection. Phosphoric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 to 7.0). The solution has a tonicity of 985 mOsm/kg; PRESERVATIVE: Benzalkonium Chloride 0.1 mg (0.01%).
Pupillary dilation following topical administration of phenylephrine hydrochloride ophthalmic solution has been demonstrated in controlled clinical studies in adults and pediatric patients with different levels of iris pigmentation. Pupil movement is generally seen within 15 minutes, maximal mydriasis between 20 to 90 minutes and recovery after 3 to 8 hours. Darker irides tend to dilate slower than lighter irides.
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% is supplied as a sterile, aqueous, topical ophthalmic solution in an opaque, white low density polyethylene (LDPE) bottle with a natural LDPE dropper tip and red cap in the following sizes:
NDC 75907-129-07 2 mL in 5 mL bottle
NDC 75907-324-22 15 mL in 15 mL bottle
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is supplied as a sterile, aqueous, topical ophthalmic solution in an opaque, white low density polyethylene (LDPE) bottle with a natural LDPE dropper tip and red cap in the following sizes:
NDC 75907-130-23 5 mL in 10 mL Bottle
After opening, Phenylephrine Hydrochloride Ophthalmic Solution, USP can be used until the expiration date on the bottle.
Keep container tightly closed.
Protect from light and excessive heat.
Do not use if solution is brown or contains precipitate.