Pilocarpine Hydrochloride
Pilocarpine Hydrochloride Prescribing Information
Regardless of the indication, the starting dose in patients with moderate hepatic impairment should be 5 mg twice daily, followed by adjustment based on therapeutic response and tolerability. Patients with mild hepatic insufficiency do not require dosage reductions. The use of pilocarpine in patients with severe hepatic insufficiency is not recommended. If needed, refer to the
Pilocarpine toxicity is characterized by an exaggeration of its parasympathomimetic effects. These may include: headache, visual disturbance, lacrimation, sweating, respiratory distress, gastrointestinal spasm, nausea, vomiting, diarrhea, atrioventricular block, tachycardia, bradycardia, hypotension, hypertension, shock, mental confusion, cardiac arrhythmia, and tremors.
The dose-related cardiovascular pharmacologic effects of pilocarpine include hypotension, hypertension, bradycardia, and tachycardia.
Pilocarpine should be administered with caution to patients with known or suspected cholelithiasis or biliary tract disease. Contractions of the gallbladder or biliary smooth muscle could precipitate complications including cholecystitis, cholangitis, and biliary obstruction.
Pilocarpine may increase ureteral smooth muscle tone and could theoretically precipitate renal colic (or "ureteral reflux"), particularly in patients with nephrolithiasis.
Cholinergic agonists may have dose-related central nervous system effects. This should be considered when treating patients with underlying cognitive or psychiatric disturbances.
Hepatic Insufficiency: Based on decreased plasma clearance observed in patients with moderate hepatic impairment, the starting dose in these patients should be 5 mg twice daily, followed by adjustment based on therapeutic response and tolerability. Patients with mild hepatic insufficiency (Child-Pugh score of 5 to 6) do not require dosage reductions. To date, pharmacokinetic studies in subjects with severe hepatic impairment (Child-Pugh score of 10 to 15) have not been carried out. The use of pilocarpine in these patients is not recommended.
Child-Pugh Scoring System for Hepatic Impairment
| * According to grading of Trey C, Burns D, and Saunders S. Treatment of hepatic coma by exchange blood transfusion. N Engl J Med. 1966; 274:473-481. | |||
Clinical and Biochemical Measurements | Points Scored for Increasing Abnormality | ||
1 | 2 | 3 | |
| Encephalopathy (grade)* | None | 1 and 2 | 3 and 4 |
| Ascites | Absent | Slight | Moderate |
| Bilirubin (mg. per 100 mL) | 1 to 2 | 2 to 3 | >3 |
| Albumin (g. per 100 mL) | 3 to 5 | 2.8 to 3.5 | <2.8 |
| Prothrombin Time (sec. Prolonged) | 1 to 4 | 4 to 6 | >6 |
| For Primary Biliary Cirrhosis:- Bilirubin (mg. per 100 mL) | 1 to 4 | 4 to 10 | >10 |
Reference: Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices.
Pilocarpine should be administered with caution to patients taking beta-adrenergic antagonists because of the possibility of conduction disturbances. Drugs with parasympathomimetic effects administered concurrently with pilocarpine would be expected to result in additive pharmacologic effects. Pilocarpine might antagonize the anticholinergic effects of drugs used concomitantly. These effects should be considered when anticholinergic properties may be contributing to the therapeutic effect of concomitant medication (e.g., atropine, inhaled ipratropium). While no formal drug interaction studies have been performed, the following concomitant drugs were used in at least 10% of patients in either or both Sjogren’s efficacy studies: acetylsalicylic acid, artificial tears, calcium, conjugated estrogens, hydroxychloroquine sulfate, ibuprofen, levothyroxine sodium, medroxyprogesterone acetate, methotrexate, multivitamins, naproxen, omeprazole, paracetamol, and prednisone.
Lifetime oral carcinogenicity studies were conducted in CD-1 mice and Sprague-Dawley rats. Pilocarpine did not induce tumors in mice at any dosage studied (up to 30 mg/kg/day, which yielded a systemic exposure approximately 50 times larger than the maximum systemic exposure observed clinically). In rats, a dosage of 18 mg/kg/day, which yielded a systemic exposure approximately 100 times larger than the maximum systemic exposure observed clinically, resulted in a statistically significant increase in the incidence of benign pheochromocytomas in both males and females, and a statistically significant increase in the incidence of hepatocellular adenomas in female rats. The tumorigenicity observed in rats was observed only at a large multiple of the maximum labeled clinical dose, and may not be relevant to clinical use.
No evidence that pilocarpine has the potential to cause genetic toxicity was obtained in a series of studies that included: 1) bacterial assays (
Oral administration of pilocarpine to male and female rats at a dosage of 18 mg/kg/day, which yielded a systemic exposure approximately 100 times larger than the maximum systemic exposure observed clinically, resulted in impaired reproductive function, including reduced fertility, decreased sperm motility, and morphologic evidence of abnormal sperm. It is unclear whether the reduction in fertility was due to effects on male animals, female animals, or both males and females. In dogs, exposure to pilocarpine at a dosage of 3 mg/kg/day (approximately 3 times the maximum recommended human dose when compared on the basis of body surface area (mg/m2) estimates) for six months resulted in evidence of impaired spermatogenesis. The data obtained in these studies suggest that pilocarpine may impair the fertility of male and female humans. Pilocarpine hydrochloride tablets should be administered to individuals who are attempting to conceive a child only if the potential benefit justifies potential impairment of fertility.
Pilocarpine was associated with a reduction in the mean fetal body weight and an increase in the incidence of skeletal variations when given to pregnant rats at a dosage of 90 mg/kg/day (approximately 26 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates). These effects may have been secondary to maternal toxicity. In another study, oral administration of pilocarpine to female rats during gestation and lactation at a dosage of 36 mg/kg/day (approximately 10 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates) resulted in an increased incidence of stillbirths; decreased neonatal survival and reduced mean body weight of pups were observed at dosages of 18 mg/kg/day (approximately 5 times the maximum recommended dose for a 50 kg human when compared on the basis of body surface area (mg/m2) estimates) and above. There are no adequate and well-controlled studies in pregnant women. Pilocarpine hydrochloride tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from pilocarpine hydrochloride tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Safety and effectiveness in pediatric patients have not been established.
Head & Neck Cancer Patients
Pilocarpine hydrochloride tablets are contraindicated in patients with uncontrolled asthma, known hypersensitivity to pilocarpine, and when miosis is undesirable, e.g., in acute iritis and in narrow-angle (angle closure) glaucoma.
Adverse Event | Pilocarpine Hydrochloride | Placebo | |
| | 10 mg t.i.d. (30 mg/day) | 5 mg t.i.d. (15 mg/day) | (t.i.d.) |
| Sweating | N=121/68% | N=141/29% | N=152/9% |
| Nausea | 15 | 6 | 4 |
| Rhinitis | 14 | 5 | 7 |
| Diarrhea | 7 | 4 | 5 |
| Chills | 15 | 3 | <1 |
| Flushing | 13 | 8 | 3 |
| Urinary Frequency | 12 | 9 | 7 |
| Dizziness | 12 | 5 | 4 |
| Asthenia | 12 | 6 | 3 |
In addition, the following adverse events (≥3% incidence) were reported at dosages of 15 to 30 mg/day in the controlled clinical trials:
Adverse Event | Pilocarpine Hydrochloride | Placebo |
| | 5 to10 mg t.i.d. (15 to 30 mg/day) | (t.i.d.) |
| Headache | N=212/11% | N=152/8% |
| Dyspepsia | 7 | 5 |
| Lacrimation | 6 | 8 |
| Edema | 5 | 4 |
| Abdominal Pain | 4 | 4 |
| Amblyopia | 4 | 2 |
| Vomiting | 4 | 1 |
| Pharyngitis | 3 | 8 |
| Hypertension | 3 | 1 |
The following events were reported with treated head and neck cancer patients at incidences of 1% to 2% at dosages of 7.5 to 30 mg/day: abnormal vision, conjunctivitis, dysphagia, epistaxis, myalgias, pruritus, rash, sinusitis, tachycardia, taste perversion, tremor, voice alteration.
The following events were reported rarely in treated head and neck cancer patients (<1%): Causal relation is unknown.
Body as a whole: body odor, hypothermia, mucous membrane abnormality
Cardiovascular: bradycardia, ECG abnormality, palpitations, syncope
Digestive: anorexia, increased appetite, esophagitis, gastrointestinal disorder, tongue disorder
Hematologic: leukopenia, lymphadenopathy
Nervous: anxiety, confusion, depression, abnormal dreams, hyperkinesia, hypesthesia, nervousness, parethesias, speech disorder, twitching
Respiratory: increased sputum, stridor, yawning
Skin: seborrhea
Special senses: deafness, eye pain, glaucoma
Urogenital: dysuria, metrorrhagia, urinary impairment
In long-term treatment were two patients with underlying cardiovascular disease of whom one experienced a myocardial infarct and another an episode of syncope. The association with drug is uncertain.
Adverse Event | Pilocarpine Hydrochloride | Placebo |
5 mg q.i.d. (20 mg/day) | (q.i.d) | |
Sweating | N=255/40% | N=253/7% |
Urinary Frequency | 10 | 4 |
Nausea | 9 | 9 |
Flushing | 9 | 2 |
Rhinitis | 7 | 8 |
Diarrhea | 6 | 7 |
Chills | 4 | 2 |
Increased Salivation | 3 | 0 |
Asthenia | 2 | 2 |
In addition, the following adverse events (≥3% incidence) were reported at dosages of 20 mg/day in the controlled clinical trials:
Adverse Event | Pilocarpine Hydrochloride | Placebo |
| 5 mg q.i.d. (20 mg/day) | (q.i.d) | |
| Headache | N=255/13% | N=253/19% |
| Flu Syndrome | 9 | 9 |
| Dyspepsia | 7 | 7 |
| Dizziness | 6 | 7 |
| Pain | 4 | 2 |
| Sinusitis | 4 | 5 |
| Abdominal Pain | 3 | 4 |
| Vomiting | 3 | 1 |
| Pharyngitis | 2 | 5 |
| Rash | 2 | 3 |
| Infection | 2 | 6 |
The following events were reported in Sjogren's patients at incidences of 1% to 2% at dosing of 20 mg/day: accidental injury, allergic reaction, back pain, blurred vision, constipation, increased cough, edema, epistaxis, face edema, fever, flatulence, glossitis, lab test abnormalities, including chemistry, hematology, and urinalysis, myalgia, palpitation, pruritus, somnolence, stomatitis, tachycardia, tinnitus, urinary incontinence, urinary tract infection, and vaginitis.
The following events were reported rarely in treated Sjogren's patients (<1%) at dosing of 10 to 30 mg/day: Causal relation is unknown.
Body as a whole: chest pain, cyst, death, moniliasis, neck pain, neck rigidity, photosensitivity reaction
Cardiovascular: angina pectoris, arrhythmia, ECG abnormality, hypotension, hypertension, intracranial hemorrhage, migraine, myocardial infarction
Digestive: anorexia, bilirubinemia, cholelithiasis, colitis, dry mouth, eructation, gastritis, gastroenteritis, gastrointestinal disorder, gingivitis, hepatitis, abnormal liver function tests, melena, nausea & vomiting, pancreatitis, parotid gland enlargement, salivary gland enlargement, sputum increased, taste loss, tongue disorder, tooth disorder
Hematologic: hematuria, lymphadenopathy, abnormal platelets, thrombocythemia, thrombocytopenia, thrombosis, abnormal WBC
Metabolic and Nutritional: peripheral edema, hypoglycemia
Musculoskeletal: arthralgia, arthritis, bone disorder, spontaneous bone fracture, pathological fracture, myasthenia, tendon disorder, tenosynovitis
Nervous: aphasia, confusion, depression, abnormal dreams, emotional lability, hyperkinesia, hypesthesia, insomnia, leg cramps, nervousness, parethesias, abnormal thinking, tremor
Respiratory: bronchitis, dyspnea, hiccup, laryngismus, laryngitis, pneumonia, viral infection, voice alteration
Skin: alopecia, contact dermatitis, dry skin, eczema, erythema nodosum, exfoliative dermatitis, herpes simplex, skin ulcer, vesiculobullous rash
Special Senses: cataract, conjunctivitis, dry eyes, ear disorder, ear pain, eye disorder, eye hemorrhage, glaucoma, lacrimation disorder, retinal disorder, taste perversion, abnormal vision
Urogenital: breast pain, dysuria, mastitis, menorrhagia, metrorrhagia, ovarian disorder, pyuria, salpingitis, urethral pain, urinary urgency, vaginal hemorrhage, vaginal moniliasis
The following adverse experiences have been reported rarely with ocular pilocarpine: A-V block, agitation, ciliary congestion, confusion, delusion, depression, dermatitis, middle ear disturbance, eyelid twitching, malignant glaucoma, iris cysts, macular hole, shock, and visual hallucination.
Pilocarpine should be administered with caution to patients taking beta-adrenergic antagonists because of the possibility of conduction disturbances. Drugs with parasympathomimetic effects administered concurrently with pilocarpine would be expected to result in additive pharmacologic effects. Pilocarpine might antagonize the anticholinergic effects of drugs used concomitantly. These effects should be considered when anticholinergic properties may be contributing to the therapeutic effect of concomitant medication (e.g., atropine, inhaled ipratropium). While no formal drug interaction studies have been performed, the following concomitant drugs were used in at least 10% of patients in either or both Sjogren’s efficacy studies: acetylsalicylic acid, artificial tears, calcium, conjugated estrogens, hydroxychloroquine sulfate, ibuprofen, levothyroxine sodium, medroxyprogesterone acetate, methotrexate, multivitamins, naproxen, omeprazole, paracetamol, and prednisone.
Pilocarpine hydrochloride tablets, USP contain pilocarpine hydrochloride, a cholinergic agonist for oral use. Pilocarpine hydrochloride is a white or almost white, crystalline powder or colorless crystals, hygroscopic which is very soluble in water and freely soluble in ethanol (96 percent). Pilocarpine hydrochloride, with a chemical name of (3
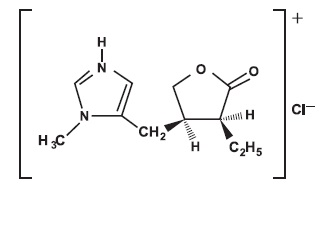
Each 5 mg pilocarpine hydrochloride tablet, USP for oral administration contains 5 mg of pilocarpine hydrochloride USP. Inactive ingredients in the tablet are: hypromellose, microcrystalline cellulose, polyethylene glycol, stearic acid and titanium dioxide.
Each 7.5 mg pilocarpine hydrochloride tablet, USP for oral administration contains 7.5 mg of pilocarpine hydrochloride USP. Inactive ingredients in the tablet are: FD&C blue # 2, hypromellose, microcrystalline cellulose, polyethylene glycol, stearic acid and titanium dioxide.
FDA approved dissolution test specifications differ from USP.