Pimozide
Pimozide Prescribing Information
Pimozide tablets, USP are indicated for the suppression of motor and phonic tics in patients with Tourette’s Disorder who have failed to respond satisfactorily to standard treatment. Pimozide Tablets, USP are not intended as a treatment of first choice nor is it intended for the treatment of tics that are merely annoying or cosmetically troublesome. Pimozide Tablets, USP should be reserved for use in Tourette’s Disorder patients whose development and/or daily life function is severely compromised by the presence of motor and phonic tics.
Evidence supporting approval of pimozide tablets, USP for use in Tourette’s Disorder was obtained in two controlled clinical investigations which enrolled patients between the ages of 8 and 53 years. Most subjects in the two trials were 12 or older.
An ECG should be done at baseline and periodically thereafter, especially during the period of dose adjustment (see WARNINGS and PRECAUTIONS - Laboratory Tests). Periodic attempts should be made to reduce the dosage of pimozide tablets, USP to see whether or not tics persist at the level and extent first identified. In attempts to reduce the dosage of pimozide tablets, USP consideration should be given to the possibility that increases of tic intensity and frequency may represent a transient, withdrawal related phenomenon rather than a return of disease symptoms. Specifically, one to two weeks should be allowed to elapse before one concludes that an increase in tic manifestations is a function of the underlying disease syndrome rather than a response to drug withdrawal. A gradual withdrawal is recommended in any case.
Treatment should be initiated at a dose of 0.05 mg/kg preferably taken once at bedtime. The dose may be increased every third day to a maximum of 0.2 mg/kg not to exceed 10 mg/day. At doses above 0.05 mg/kg/day, CYP 2D6 genotyping should be performed. In poor CYP 2D6 metabolizers, pimozide tablets, USP doses should not exceed 0.05 mg/kg/day, and doses should not be increased earlier than 14 days (see
In general, treatment with pimozide tablets, USP should be initiated with a dose of 1 to 2 mg a day in divided doses. The dose may be increased thereafter every other day. Most patients are maintained at less than 0.2 mg/kg/day, or 10 mg/day, whichever is less. Doses greater than 0.2 mg/kg/day or 10 mg/day are not recommended.
At doses above 4 mg/day, CYP 2D6 genotyping should be performed. In poor CYP 2D6 metabolizers, pimozide tablets, USP doses should not exceed 4 mg/day, and doses should not be increased earlier than 14 days (see
1. Pimozide is contraindicated in the treatment of simple tics or tics other than those associated with Tourette’s Disorder.
2. Pimozide should not be used in patients taking drugs that may, themselves, cause motor and phonic tics (e.g., pemoline, methylphenidate and amphetamines) until such patients have been withdrawn from these drugs to determine whether or not the drugs, rather than Tourette’s Disorder, are responsible for the tics.
3. Because pimozide prolongs the QT interval of the electrocardiogram it is contraindicated in patients with congenital long QT syndrome, patients with a history of cardiac arrhythmias, patients taking other drugs which prolong the QT interval of the electrocardiogram or patients with known hypokalemia or hypomagnesemia (see also
4. Pimozide is contraindicated in patients with severe toxic central nervous system depression or comatose states from any cause.
5. Pimozide is contraindicated in patients with hypersensitivity to it. As it is not known whether cross-sensitivity exists among the antipsychotics, pimozide should be used with appropriate caution in patients who have demonstrated hypersensitivity to other antipsychotic drugs.
6. Ventricular arrhythmias have been rarely associated with the use of macrolide antibiotics in patients with prolonged QT intervals, as might be produced by pimozide. Specifically, two sudden deaths have been reported when clarithromycin was added to ongoing pimozide therapy. Furthermore, some evidence suggests that pimozide is metabolized partly by the enzyme system cytochrome P450 3A4 (CYP 3A4). Macrolide antibiotics are inhibitors of CYP 3A4, and thus could potentially impede pimozide metabolism. For these reasons, pimozide is contraindicated in patients receiving the macrolide antibiotics clarithromycin, erythromycin, azithromycin, dirithromycin, and troleandomycin.
7. Concomitant use in patients taking Celexa or Lexapro is contraindicated (see
8. Clinical drug interaction studies have demonstrated that pimozide is also metabolized by CYP 2D6. Concomitant use of pimozide with paroxetine and other strong CYP 2D6 inhibitors is contraindicated (See
9. Concomitant use of pimozide in patients taking sertraline is contraindicated (See
Because azole antifungal agents are also inhibitors of the CYP 3A4 enzymes and thus may likewise impair pimozide metabolism, pimozide is contraindicated in patients receiving the azole antifungal agents itraconazole and ketoconazole.
Similarly, protease inhibitor drugs are also inhibitors of CYP 3A4, and thus pimozide is contraindicated in patients receiving protease inhibitors such as ritonavir, saquinovir, indinavir, and nelfinavir. (See
Nefazodone is a potent inhibitor of CYP 3A4, and its concomitant use with pimozide is also contraindicated.
Other drugs that are relatively less potent inhibitors of CYP 3A4 should also be avoided, in view of the risks: e.g., zileuton, fluvoxamine.
Other types of neuromuscular reactions (motor restlessness, dystonia, akathisia, hyperreflexia, opisthotonos, oculogyric crises) have been reported far less frequently. Severe extrapyramidal reactions have been reported to occur at relatively low doses. Generally the occurrence and severity of most extrapyramidal symptoms are dose-related since they occur at relatively high doses and have been shown to disappear or become less severe when the dose is reduced. Administration of antiparkinson drugs such as benztropine mesylate or trihexyphenidyl hydrochloride may be required for control of such reactions. It should be noted that persistent extrapyramidal reactions have been reported and that the drug may have to be discontinued in such cases.
However, some patients on maintenance treatment experience transient dyskinetic signs after abrupt withdrawal. In certain of these cases the dyskinetic movements are indistinguishable from the syndrome described below under “Tardive Dyskinesia” except for duration. It is not known whether gradual withdrawal of antipsychotic drugs will reduce the rate of occurrence of withdrawal emergent neurological signs, but until further evidence becomes available, it seems reasonable to gradually withdraw use of pimozide.
There is no known effective treatment for tardive dyskinesia; antiparkinson agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, this syndrome may be masked.
It has been reported that fine vermicular movement of the tongue may be an early sign of tardive dyskinesia and if the medication is stopped at that time the syndrome may not develop.
Electrocardiographic Changes:
Neuroleptic Malignant Syndrome:
The following adverse reaction tabulation was derived from 20 patients in a 6-week long placebo-controlled clinical trial of pimozide in Tourette’s Disorder.
| Body System/ Adverse Reaction | Pimozide (N=20) | Placebo (N=20) |
| Body as a Whole | | |
| Headache | 1 | 2 |
| Gastrointestinal | | |
| Dry Mouth | 5 | 1 |
| Diarrhea | 1 | 0 |
| Nausea | 0 | 2 |
| Vomiting | 0 | 1 |
| Constipation | 4 | 2 |
| Eructations | 0 | 1 |
| Thirsty | 1 | 0 |
| Appetite increase | 1 | 0 |
| Endocrine | | |
| Menstrual disorder | 0 | 1 |
| Breast secretions | 0 | 1 |
| Musculoskeletal | | |
| Muscle cramps | 0 | 1 |
| Muscle tightness | 3 | 0 |
| Stooped posture | 2 | 0 |
| CNS | | |
| Drowsiness | 7 | 3 |
| Sedation | 14 | 5 |
| Insomnia | 2 | 2 |
| Dizziness | 0 | 1 |
| Akathisia | 8 | 0 |
| Rigidity | 2 | 0 |
| Speech disorder | 2 | 0 |
| Handwriting change | 1 | 0 |
| Akinesia | 8 | 0 |
| Psychiatric | | |
| Depression | 2 | 3 |
| Excitement | 0 | 1 |
| Nervous | 1 | 0 |
| Adverse behavior effect | 5 | 0 |
| Special Senses | | |
| Visual disturbance | 4 | 0 |
| Taste change | 1 | 0 |
| Sensitivity of eyes to light | 1 | 0 |
| Decrease accommodation | 4 | 1 |
| Spots before eyes | 0 | 1 |
| Urogenital | | |
| Impotence | 3 | 0 |
The following adverse event tabulation was derived from 36 children (age 2 to 12) in a 24-week open trial of pimozide in Tourette’s Disorder. Because clinical investigational experience with pimozide in Tourette’s Disorder is limited, uncommon
| Body System/ Adverse Reaction | Number of Patients Experiencing Each Event (%) | |
| | All Events (N=36) | Drug-Related Events (N=36) |
| Body as a Whole | | |
| Asthenia | 9 (25.0) | 5 (13.8) |
| Headache | 8 (22.2) | 1 (2.7) |
| Gastrointestinal | | |
| Dysphagia | 1 (2.7) | 1 (2.7) |
| Increased Salivation | 5 (13.8) | 2 (5.5) |
| Musculoskeletal | | |
| Myalgia | 1 (2.7) | 1 (2.7) |
| Central Nervous System | | |
| Dreaming Abnormal | 1 (2.7) | 1 (2.7) |
| Hyperkinesia | 2 (5.5) | 1 (2.7) |
| Somnolence | 10 (27.7) | 9 (25.0) |
| Torticollis | 1 (2.7) | 1 (2.7) |
| Tremor, Limbs | 1 (2.7) | 1 (2.7) |
| Psychiatric | | |
| Adverse Behavior Effect | 10 (27.7) | 8 (22.2) |
| Nervous | 3 (8.3) | 2 (5.5) |
| Skin | | |
| Rash | 3 (8.3) | 1 (2.7) |
| Special Senses | | |
| Visual Disturbance | 2 (5.5) | 1 (2.7) |
| Cardiovascular | | |
| ECG Abnormal | 1 (2.7) | 1 (2.7) |
adverse reactions may not have been detected. The physician should consider that other adverse reactions associated with antipsychotics may occur.
In addition to the adverse reactions listed above, those listed below have been reported in U.S. clinical trials of pimozide in conditions other than Tourette’s Disorder.
Cardiovascular/Respiratory:
The following experiences were described in spontaneous postmarketing reports. These reports do not provide sufficient information to establish a clear causal relationship with the use of pimozide.
Because pimozide prolongs the QT interval of the electrocardiogram, an additive effect on QT interval would be anticipated if administered with other drugs, such as phenothiazines, tricyclic antidepressants or antiarrhythmic agents, which prolong the QT interval. Accordingly, pimozide should not be given with dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol, tacrolimus, ziprasidone, or other drugs that have demonstrated QT prolongation as one of their pharmacodynamic effects. Also, the use of macrolide antibiotics in patients with prolonged QT intervals has been rarely associated with ventricular arrhythmias. Such concomitant administration should not be undertaken (see
Since pimozide is partly metabolized via CYP 3A4, it should not be administered concomitantly with inhibitors of this metabolic system, such as azole antifungal agents and protease inhibitor drugs (see
Pimozide and Celexa: In a controlled study, a single dose of pimozide 2 mg coadministered with racemic citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately 10 msec compared to pimozide given alone. Racemic citalopram did not alter the mean AUC or Cmax of pimozide. The mechanism of this pharmacodynamic interaction is not known. Concomitant use of Pimozide and Celexa or Lexapro is contraindicated (See
CYP 2D6 inhibitors: In healthy subjects, co-administration of pimozide 2 mg (single dose) and paroxetine 60 mg resulted in a 151% increase in pimozide AUC and a 62% increase in pimozide Cmax compared to pimozide administered alone. The increase in pimozide AUC and Cmax is related to the CYP 2D6 inhibitory properties of paroxetine. Concomitant use of pimozide and paroxetine or other strong CYP 2D6 inhibitors are contraindicated (see
As CYP 1A2 may also contribute to the metabolism of pimozide, prescribers should be aware of the theoretical potential for drug interactions with inhibitors of this enzymatic system.
Pimozide may be capable of potentiating CNS depressants, including analgesics, sedatives, anxiolytics, and alcohol.
Rare case reports have suggested possible additive effects of pimozide and fluoxetine leading to bradycardia.
Concomitant administration of pimozide and sertraline should be contraindicated (See
Patients should avoid grapefruit juice because it may inhibit the metabolism of pimozide by CYP 3A4.
Pimozide is an orally active antipsychotic agent of the diphenyl-butylpiperidine series. The structural formula of pimozide, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazole-2-one is:
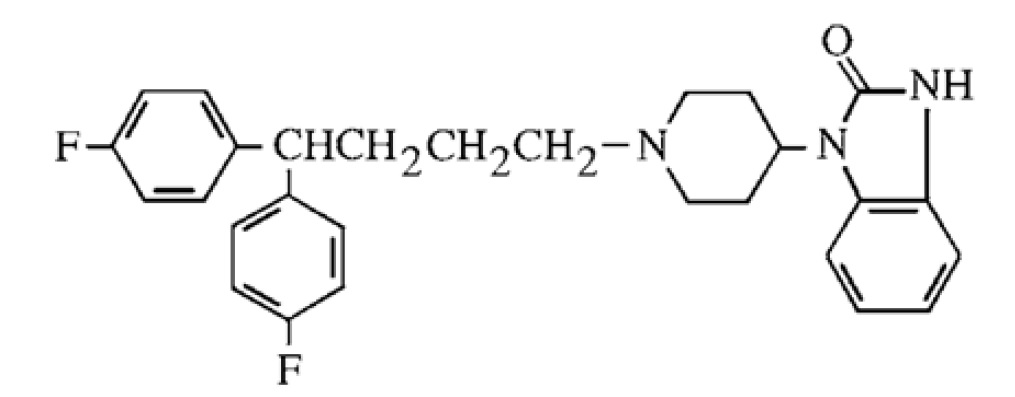
The solubility of pimozide in water is less than 0.01 mg/mL; it is slightly soluble in most organic solvents.
Each white pimozide tablet contains either 1 mg or 2 mg of pimozide and the following inactive ingredients: calcium stearate, microcrystalline cellulose, lactose anhydrous and corn starch.