Podofilox
Podofilox Prescribing Information
Podofilox gel is indicated for the topical treatment of anogenital warts (external genital warts and perianal warts). This product is not indicated in the treatment of mucous membrane warts (see
Although anogenital warts have a characteristic appearance, histopathologic confirmation should be obtained if there is any doubt of the diagnosis. Differentiating warts from squamous cell carcinoma and "Bowenoid papulosis" is of particular concern. Squamous cell carcinoma may also be associated with human papillomavirus which should not be treated with podofilox gel.
The prescriber should ensure that the patient is fully aware of the correct method of therapy and identify which specific warts should be treated.
Apply twice daily for 3 consecutive days, then discontinue for 4 consecutive days. This one-week cycle of treatment may be repeated until there is no visible wart tissue or for a maximum of four cycles.
Podofilox gel should be applied to the warts with the applicator tip or finger. Application on the surrounding normal tissue should be minimized.
Care should be taken to allow the gel to dry before allowing the return of opposing skin surfaces to their normal positions. Patients should be instructed to wash their hands thoroughly before and after each application.
Podofilox gel is contraindicated for patients who develop hypersensitivity or intolerance to any components of the formulation.
In clinical trials with podofilox gel, the following local adverse reactions were reported during the treatment of anogenital warts. The severity of local adverse reactions were predominantly mild or moderate and did not increase during the treatment period. Severe reactions were most frequent within the first 2 weeks of treatment.
Adverse Reaction | Mild | Moderate | Severe |
Inflammation | 32.2% | 30.4% | 9.3% |
Burning | 37.1% | 25.9% | 11.5% |
Erosion | 27.0% | 20.8% | 8.9% |
Pain | 23.7% | 20.4% | 11.5% |
Itching | 32.2% | 16.0% | 7.8% |
Bleeding | 19.2% | 3.0% | 0.7% |
Other local adverse reactions reported included stinging (7%), and erythema (5%); less commonly reported local adverse events included desquamation, scabbing, discoloration, tenderness, dryness, crusting, fissures, soreness, ulceration, swelling/edema, tingling, rash, and blisters.
The most common systemic adverse event reported during the clinical studies was headache (7%).
Podofilox is an antimitotic drug which can be chemically synthesized or purified from the plant families
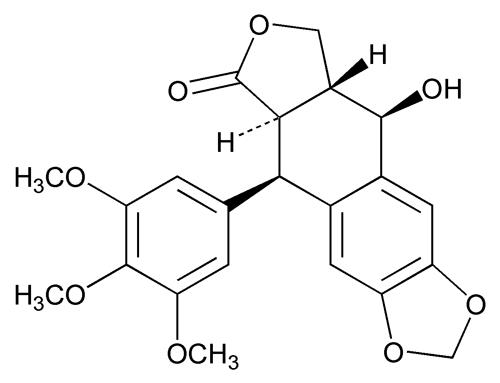
Podofilox has a molecular weight of 414.4 daltons, and is soluble in alcohol and sparingly soluble in water. Its chemical name is [5R,-(5α, 5aβ, 8aα, 9α]-5,8,8a,9-tetrahydro-9-hydroxy- 5-(3,4,5-trimethoxyphenyl) furo[3',4':6,7]naphtho-[2,3,-d]-1,3-dioxol-6(5aH)-one.
Podofilox has the following structural formula:
Treatment of anogenital warts with podofilox results in necrosis of visible wart tissue. The exact mechanism of action is unknown.
In systemic absorption studies in 52 patients, topical application of 0.05 mL of an ethanolic solution containing 0.5% podofilox to external genitalia did not result in detectable serum levels. Applications of 0.1 to 1.5 mL resulted in peak serum levels of 1 to 17 ng/mL one to two hours after application. The elimination half-life ranged from 1.0 to 4.5 hours. The drug was not found to accumulate after multiple treatments1.