Potassium Phosphates
(Potassium Phosphate, Monobasic Potassium Phosphate, Dibasic Injection,)Potassium Phosphates Prescribing Information
Potassium phosphates injection is indicated as a source of phosphorus:
- in intravenous fluids to correct hypophosphatemia in adults and pediatric patients when oral or enteral replacement is not possible, insufficient or contraindicated.
- for parenteral nutrition in adults and pediatric patients when oral or enteral nutrition is not possible, insufficient or contraindicated.
Potassium phosphates injection, USP is a sterile clear, colorless solution supplied as:
- phosphorus 15 mmol/5 mL (3 mmol/mL) and potassium 22 mEq/5 mL (4.4 mEq /mL) in a single-dose vial.
- phosphorus 45 mmol/15 mL (3 mmol/mL) and potassium 66 mEq/15 mL (4.4 mEq/mL) in a single-dose vial.
- phosphorus 150 mmol/50 mL (3 mmol/mL) and potassium 220 mEq/50 mL (4.4 mEq/mL) in Pharmacy Bulk Package vial.
Potassium phosphates injection is contraindicated in patients with:
- Hyperkalemia [see Warning and Precautions (5.3)].
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m2) or end stage renal disease [see Warning and Precautions (5.3)].
- Hyperphosphatemia [see Warning and Precautions (5.4)].
- Hypercalcemia or significant hypocalcemia [see Warning and Precautions (5.4)].
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Aluminum Toxicity [see Warnings and Precautions (5.5)]
- Hypomagnesemia [see Warnings and Precautions (5.6)]
- Vein Damage and Thrombosis [see Warnings and Precautions (5.7)]
The following adverse reactions in Table 5 have been reported in clinical studies or post-marketing reports in patients receiving intravenously administered potassium phosphates. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
System Organ Class | Adverse Reactions |
Metabolism and Nutrition Disorders | pulmonary embolism due to pulmonary vascular precipitates [see Warnings and Precautions (5.2)], hyperkalemia [see Warnings and Precautions (5.3)] , hyperphosphatemia [see Warnings and Precautions (5.4)], hypocalcemia [see Warnings and Precautions (5.5)] , hypovolemia and osmotic diuresis |
Cardiac Disorders | hypotension, arrhythmia, heart block, cardiac arrest, bradycardia, chest pain, ECG changes [see Warnings and Precautions (5.1)] and edema |
Respiratory, Thoracic and Mediastinal Disorders | dyspnea [see Warnings and Precautions (5.2)] |
Renal and Urinary Disorders | acute phosphate nephropathy (i.e., nephrocalcinosis with acute kidney injury), decreased urine output and transition to chronic kidney disease [see Warnings and Precautions (5.4)] |
Gastrointestinal Disorders | diarrhea, stomach pain |
Musculoskeletal and Connective Tissue Disorders | weakness |
Nervous System Disorders | confusion, lethargy, paralysis, paresthesia |
Potassium Phosphates Injection, USP, a phosphorus replacement product containing phosphorus 3 mmol/mL and potassium 4.4 mEq/mL. It is a sterile clear, colorless, non-pyrogenic, concentrated solution containing a mixture of monobasic potassium phosphate, USP and dibasic potassium phosphate, USP in water for injection. It is supplied as a 5 mL and 15 mL single-dose vials and a 50 mL Pharmacy Bulk Package vial.
Monobasic potassium phosphate, USP is chemically designated potassium dihydrogen phosphate. The molecular formula is KH2PO4, molecular weight is 136.084 g/mol and the structural formula is as below:
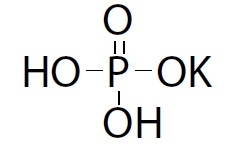
It appears as colorless crystals or white granular or crystalline powder. It is freely soluble in water and practically insoluble in alcohol.
Dibasic potassium phosphate, USP is chemically designated dipotassium hydrogen phosphate. The molecular formula is K2HPO4, molecular weight is 174.18 g/mol and the structural formula is as below:
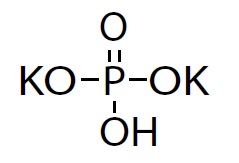
It appears as colorless crystals or white granular or crystalline powder. It is freely soluble in water.
Each mL of Potassium Phosphates Injection, USP contains monobasic potassium phosphate USP, 224 mg; dibasic potassium phosphate USP, 236 mg and water for injection.
Each mL contains 3 mmol phosphorus (equivalent to 93 mg phosphorus) and 4.4 mEq potassium (equivalent to 170 mg of potassium). Note: 1 mmol of phosphorus is equal to 1 mmol phosphate. The pH is 6.0 to 7.0.
This product contains no more than 900 mcg/L of aluminum
The osmolarity is 7.4 mOsmol/mL (calc).
The solution is administered after dilution or admixing by the intravenous route.
Potassium Phosphates Injection, USP is a sterile clear, colorless solution supplied as phosphorus 3 mmol/mL and potassium 4.4 mEq/mL as shown:
Product Code | Unit of Sale | Strength | Each |
1693 | NDC 80830-1693-5 Carton containing 25 units | Phosphorus 15 mmol/5 mL and Potassium 22 mEq/5 mL | NDC 80830-1693-1 5 mL single-dose, polypropylene vial |
NDC 80830-1693-3 Carton containing 5 units | |||
1691 | NDC 80830-1691-5 Carton containing 25 units | Phosphorus 45 mmol/15 mL and Potassium 66 mEq/15 mL | NDC 80830-1691-1 15 mL single-dose, polypropylene vial |
NDC 80830-1691-2 Carton containing 10 units | |||
1692 | NDC 80830-1692-5 Carton containing 25 units | Phosphorus 150 mmol/50 mL and Potassium 220 mEq/50 mL | NDC 80830-1692-1 50 mL fill Pharmacy Bulk Package, polypropylene vial |
NDC 80830-1692-2 Carton containing 10 units |
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Pharmacy Bulk Package vial: Discard within 4 hours of initial entry
For storage of admixed solution,