Prasugrel Prescribing Information
- Prasugrel can cause significant, sometimes fatal, bleeding[see.,
5.1 General Risk of BleedingThienopyridines, including prasugrel, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL), bleeding events were more common on prasugrel than on clopidogrel
[see Adverse Reactions (6.1)]. The bleeding risk is highest initially, as shown in Figure 1 (events through 450 days; inset shows events through 7 days).
Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events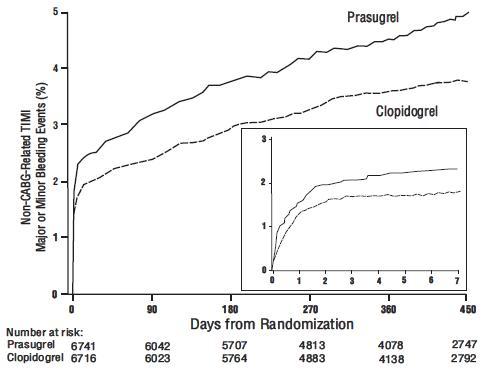
Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding.
Do not use prasugrel in patients with active bleeding, prior TIA or stroke[see Contraindications (4.1, 4.2)].
Other risk factors for bleeding are:- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Adverse Reactions (6.1), Use in Specific Populations (8.5), Clinical Pharmacology (12.3), and Clinical Studies (14)].
- CABG or other surgical procedure[see Warnings and Precautions (5.2)].
- Body weight <60 kg. Consider a lower (5 mg) maintenance dose[see Dosage and Administration (2), Adverse Reactions (6.1), and Use in Specific Populations (8.6)].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment)[see Adverse Reactions (6.1)and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs], and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38[see Drug Interactions (7.1, 7.2, 7.4) and Clinical Studies (14)].
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel’s active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.

Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events. and5.2 Coronary Artery Bypass Graft Surgery-Related BleedingThe risk of bleeding is increased in patients receiving prasugrel who undergo CABG. If possible, prasugrel should be discontinued at least 7 days prior to CABG.
Of the 437 patients who underwent CABG during TRITON-TIMI 38, the rates of CABG-related TIMI Major or Minor bleeding were 14.1% in the prasugrel group and 4.5% in the clopidogrel group[see Adverse Reactions (6.1)]. The higher risk for bleeding events in patients treated with prasugrel persisted up to 7 days from the most recent dose of study drug. For patients receiving a thienopyridine within 3 days prior to CABG, the frequencies of TIMI Major or Minor bleeding were 26.7% (12 of 45 patients) in the prasugrel group, compared with 5.0% (3 of 60 patients) in the clopidogrel group. For patients who received their last dose of thienopyridine within 4 to 7 days prior to CABG, the frequencies decreased to 11.3% (9 of 80 patients) in the prasugrel group and 3.4% (3 of 89 patients) in the clopidogrel group.
Do not start prasugrel in patients likely to undergo urgent CABG. CABG-related bleeding may be treated with transfusion of blood products, including packed red blood cells and platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.]6.1 Clinical Trials ExperienceSafety in patients with ACS undergoing PCI was evaluated in a clopidogrel-controlled study, TRITON-TIMI 38, in which 6741 patients were treated with prasugrel (60 mg loading dose and 10 mg once daily) for a median of 14.5 months (5802 patients were treated for over 6 months; 4136 patients were treated for more than 1 year). The population treated with prasugrel was 27 to 96 years of age, 25% female, and 92% Caucasian. All patients in the TRITON-TIMI 38 study were to receive aspirin. The dose of clopidogrel in this study was a 300 mg loading dose and 75 mg once daily.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with the rates observed in other clinical trials of another drug and may not reflect the rates observed in practice.Drug Discontinuation
The rate of study drug discontinuation because of adverse reactions was 7.2% for prasugrel and 6.3% for clopidogrel. Bleeding was the most common adverse reaction leading to study drug discontinuation for both drugs (2.5% for prasugrel and 1.4% for clopidogrel).BleedingIn TRITON-TIMI 38, overall rates of TIMI Major or Minor bleeding adverse reactions unrelated to coronary artery bypass graft surgery (CABG) were significantly higher on prasugrel than on clopidogrel, as shown in Table 1.
Bleeding Unrelated to CABG SurgeryTable 1: Non-CABG-Related Bleeding*(TRITON-TIMI 38) *Patients may be counted in more than one row.
†See 5.1for definition.Prasugrel (%)(N=6741)Clopidogrel (%)(N=6716)TIMI Major or Minor bleeding 4.5 3.4 TIMI Major bleeding† 2.2 1.7 Life-threatening 1.3 0.8 Fatal 0.3 0.1 Symptomatic intracranial hemorrhage (ICH) 0.3 0.3 Requiring inotropes 0.3 0.1 Requiring surgical intervention 0.3 0.3 Requiring transfusion (≥4 units) 0.7 0.5 TIMI Minor bleeding† 2.4 1.9
Figure 1 demonstrates non-CABG-related TIMI Major or Minor bleeding. The bleeding rate is highest initially, as shown in Figure 1 (inset: Days 0 to 7)[see Warnings and Precautions (5.1)].Bleeding by Weight and Age
In TRITON-TIMI 38, non-CABG-related TIMI Major or Minor bleeding rates in patients with the risk factors of age ≥75 years and weight <60 kg are shown in Table 2.Table 2: Bleeding Rates for Non-CABG-Related Bleeding by Weight and Age (TRITON-TIMI 38) *10 mg prasugrel maintenance dose
†75 mg clopidogrel maintenance doseMajor/MinorFatalPrasugrel*(%)Clopidogrel†(%)Prasugrel*(%)Clopidogrel†(%)Weight <60 kg (N=308 prasugrel, N=356 clopidogrel) 10.1 6.5 0.0 0.3 Weight ≥60 kg (N=6373 prasugrel, N=6299 clopidogrel) 4.2 3.3 0.3 0.1 Age <75 years (N=5850 prasugrel, N=5822 clopidogrel) 3.8 2.9 0.2 0.1 Age ≥75 years (N=891 prasugrel, N=894 clopidogrel) 9.0 6.9 1.0 0.1 Bleeding Related to CABGIn TRITON-TIMI 38, 437 patients who received a thienopyridine underwent CABG during the course of the study. The rate of CABG-related TIMI Major or Minor bleeding was 14.1% for the prasugrel group and 4.5% in the clopidogrel group (see Table 3). The higher risk for bleeding adverse reactions in patients treated with prasugrel persisted up to 7 days from the most recent dose of study drug.Table 3: CABG-Related Bleeding*(TRITON-TIMI 38) *Patients may be counted in more than one row. Prasugrel (%)(N=213)Clopidogrel (%)(N=224)TIMI Major or Minor bleeding 14.1 4.5 TIMI Major bleeding 11.3 3.6 Fatal 0.9 0 Reoperation 3.8 0.5 Transfusion of ≥5 units 6.6 2.2 Intracranial hemorrhage 0 0 TIMI Minor bleeding 2.8 0.9 Bleeding Reported as Adverse ReactionsHemorrhagic events reported as adverse reactions in TRITON-TIMI 38 were, for prasugrel and clopidogrel, respectively: epistaxis (6.2%, 3.3%), gastrointestinal hemorrhage (1.5%, 1.0%), hemoptysis (0.6%, 0.5%), subcutaneous hematoma (0.5%, 0.2%), post-procedural hemorrhage (0.5%, 0.2%), retroperitoneal hemorrhage (0.3%, 0.2%), pericardial effusion/hemorrhage/tamponade (0.3%, 0.2%), and retinal hemorrhage (0.0%, 0.1%).Malignancies
During TRITON-TIMI 38, newly diagnosed malignancies were reported in 1.6% and 1.2% of patients treated with prasugrel and clopidogrel, respectively. The sites contributing to the differences were primarily colon and lung. In another Phase 3 clinical study of ACS patients not undergoing PCI, in which data for malignancies were prospectively collected, newly diagnosed malignancies were reported in 1.8% and 1.7% of patients treated with prasugrel and clopidogrel, respectively. The site of malignancies was balanced between treatment groups except for colorectal malignancies. The rates of colorectal malignancies were 0.3% prasugrel, 0.1% clopidogrel and most were detected during investigation of GI bleed or anemia. It is unclear if these observations are causally related, are the result of increased detection because of bleeding, or are random occurrences.Other Adverse Events
In TRITON-TIMI 38, common and other important nonhemorrhagic adverse events were, for prasugrel and clopidogrel, respectively: severe thrombocytopenia (0.06%, 0.04%), anemia (2.2%, 2.0%), abnormal hepatic function (0.22%, 0.27%), allergic reactions (0.36%, 0.36%), and angioedema (0.06%, 0.04%). Table 4 summarizes the adverse events reported by at least 2.5% of patients.Table 4: Non-Hemorrhagic Treatment Emergent Adverse Events Reported by at Least 2.5% of Patients in Either Group Prasugrel (%)(N=6741)Clopidogrel (%)(N=6716)Hypertension 7.5 7.1 Hypercholesterolemia/Hyperlipidemia 7.0 7.4 Headache 5.5 5.3 Back pain 5.0 4.5 Dyspnea 4.9 4.5 Nausea 4.6 4.3 Dizziness 4.1 4.6 Cough 3.9 4.1 Hypotension 3.9 3.8 Fatigue 3.7 4.8 Noncardiac chest pain 3.1 3.5 Atrial fibrillation 2.9 3.1 Bradycardia 2.9 2.4 Leukopenia (<4 x 109WBC*/L) 2.8 3.5 Rash 2.8 2.4 Pyrexia 2.7 2.2 Peripheral edema 2.7 3.0 Pain in extremity 2.6 2.6 Diarrhea 2.3 2.6 *WBC = white blood cell
- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered
- Do not use prasugrel in patients with active pathological bleeding or a history of transient ischemic attack (TIA) or stroke[see.,
4.1 Active BleedingPrasugrel tablets are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage (ICH)
[see Warnings and Precautions (5.1)and Adverse Reactions (6.1)].]4.2 Prior Transient Ischemic Attack or StrokePrasugrel tablets are contraindicated in patients with a history of prior transient ischemic attack (TIA) or stroke. In TRITON-TIMI 38 (
TRial to AssessImprovement inTherapeutic Outcomes byOptimizing Platelet InhibitioNwith Prasugrel), patients with a history of TIA or ischemic stroke (>3 months prior to enrollment) had a higher rate of stroke on prasugrel tablets (6.5%; of which 4.2% were thrombotic stroke and 2.3% were intracranial hemorrhage [ICH]) than on clopidogrel (1.2%; all thrombotic). In patients without such a history, the incidence of stroke was 0.9% (0.2% ICH) and 1.0% (0.3% ICH) with prasugrel tablets and clopidogrel, respectively. Patients with a history of ischemic stroke within 3 months of screening and patients with a history of hemorrhagic stroke at any time were excluded from TRITON-TIMI 38. Patients who experience a stroke or TIA while on prasugrel tablets generally should have therapy discontinued[see Adverse Reactions (6.1)and Clinical Studies (14)]. - In patients ≥75 years of age, prasugrel is generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior myocardial infarction [MI]) where its effect appears to be greater and its use may be considered[see.]
8.5 Geriatric UseIn TRITON-TIMI 38, 38.5% of patients were ≥65 years of age and 13.2% were ≥75 years of age. The risk of bleeding increased with advancing age in both treatment groups, although the relative risk of bleeding (prasugrel compared with clopidogrel) was similar across age groups.
Patients ≥75 years of age who received prasugrel 10 mg had an increased risk of fatal bleeding events (1.0%) compared to patients who received clopidogrel (0.1%). In patients ≥75 years of age, symptomatic intracranial hemorrhage occurred in 7 patients (0.8%) who received prasugrel and in 3 patients (0.3%) who received clopidogrel. Because of the risk of bleeding, and because effectiveness is uncertain in patients ≥75 years of age[see Clinical Studies (14)], use of prasugrel is generally not recommended in these patients, except in high-risk situations (diabetes and past history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Warnings and Precautions (5.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. - Do not start prasugrel in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue prasugrel at least 7 days prior to any surgery[see.]
5.2 Coronary Artery Bypass Graft Surgery-Related BleedingThe risk of bleeding is increased in patients receiving prasugrel who undergo CABG. If possible, prasugrel should be discontinued at least 7 days prior to CABG.
Of the 437 patients who underwent CABG during TRITON-TIMI 38, the rates of CABG-related TIMI Major or Minor bleeding were 14.1% in the prasugrel group and 4.5% in the clopidogrel group[see Adverse Reactions (6.1)]. The higher risk for bleeding events in patients treated with prasugrel persisted up to 7 days from the most recent dose of study drug. For patients receiving a thienopyridine within 3 days prior to CABG, the frequencies of TIMI Major or Minor bleeding were 26.7% (12 of 45 patients) in the prasugrel group, compared with 5.0% (3 of 60 patients) in the clopidogrel group. For patients who received their last dose of thienopyridine within 4 to 7 days prior to CABG, the frequencies decreased to 11.3% (9 of 80 patients) in the prasugrel group and 3.4% (3 of 89 patients) in the clopidogrel group.
Do not start prasugrel in patients likely to undergo urgent CABG. CABG-related bleeding may be treated with transfusion of blood products, including packed red blood cells and platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective. - Additional risk factors for bleeding include: body weight <60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding (e.g., warfarin, heparin, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs])[see.]
5.1 General Risk of BleedingThienopyridines, including prasugrel, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL), bleeding events were more common on prasugrel than on clopidogrel
[see Adverse Reactions (6.1)]. The bleeding risk is highest initially, as shown in Figure 1 (events through 450 days; inset shows events through 7 days).
Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events
Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding.
Do not use prasugrel in patients with active bleeding, prior TIA or stroke[see Contraindications (4.1, 4.2)].
Other risk factors for bleeding are:- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Adverse Reactions (6.1), Use in Specific Populations (8.5), Clinical Pharmacology (12.3), and Clinical Studies (14)].
- CABG or other surgical procedure[see Warnings and Precautions (5.2)].
- Body weight <60 kg. Consider a lower (5 mg) maintenance dose[see Dosage and Administration (2), Adverse Reactions (6.1), and Use in Specific Populations (8.6)].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment)[see Adverse Reactions (6.1)and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs], and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38[see Drug Interactions (7.1, 7.2, 7.4) and Clinical Studies (14)].
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel’s active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.

Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events. - Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of prasugrel[see.]
5.1 General Risk of BleedingThienopyridines, including prasugrel, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL), bleeding events were more common on prasugrel than on clopidogrel
[see Adverse Reactions (6.1)]. The bleeding risk is highest initially, as shown in Figure 1 (events through 450 days; inset shows events through 7 days).
Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events
Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding.
Do not use prasugrel in patients with active bleeding, prior TIA or stroke[see Contraindications (4.1, 4.2)].
Other risk factors for bleeding are:- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Adverse Reactions (6.1), Use in Specific Populations (8.5), Clinical Pharmacology (12.3), and Clinical Studies (14)].
- CABG or other surgical procedure[see Warnings and Precautions (5.2)].
- Body weight <60 kg. Consider a lower (5 mg) maintenance dose[see Dosage and Administration (2), Adverse Reactions (6.1), and Use in Specific Populations (8.6)].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment)[see Adverse Reactions (6.1)and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs], and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38[see Drug Interactions (7.1, 7.2, 7.4) and Clinical Studies (14)].
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel’s active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.

Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events. - Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered
- If possible, manage bleeding without discontinuing prasugrel. Discontinuing prasugrel, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular (CV) events[see.]
5.3 Discontinuation of PrasugrelDiscontinue thienopyridines, including prasugrel, for active bleeding, elective surgery, stroke, or TIA. The optimal duration of thienopyridine therapy is unknown. In patients who are managed with PCI and stent placement, premature discontinuation of any antiplatelet medication, including thienopyridines, conveys an increased risk of stent thrombosis, myocardial infarction, and death. Patients who require premature discontinuation of a thienopyridine will be at increased risk for cardiac events. Lapses in therapy should be avoided, and if thienopyridines must be temporarily discontinued because of an adverse event(s), they should be restarted as soon as possible
[see Contraindications (4.1, 4.2)and Warnings and Precautions (5.1)].
Prasugrel tablets are a P2Y12 platelet inhibitor indicated for the reduction of thrombotic cardiovascular events (including stent thrombosis) in patients with acute coronary syndrome who are to be managed with percutaneous coronary intervention (PCI) as follows:
- Patients with unstable angina or non-ST-elevation myocardial infarction (NSTEMI) .
1.1 Acute Coronary SyndromePrasugrel tablets are indicated to reduce the rate of thrombotic CV events (including stent thrombosis) in patients with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI) as follows:
- Patients with unstable angina (UA) or non-ST-elevation myocardial infarction (NSTEMI).
- Patients with ST-elevation myocardial infarction (STEMI) when managed with primary or delayed PCI.
Prasugrel tablets have been shown to reduce the rate of a combined endpoint of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke compared to clopidogrel. The difference between treatments was driven predominantly by MI, with no difference on strokes and little difference on CV death
[seeClinical Studies (14)]. - Patients with ST-elevation myocardial infarction (STEMI) when managed with either primary or delayed PCI .
1.1 Acute Coronary SyndromePrasugrel tablets are indicated to reduce the rate of thrombotic CV events (including stent thrombosis) in patients with acute coronary syndrome (ACS) who are to be managed with percutaneous coronary intervention (PCI) as follows:
- Patients with unstable angina (UA) or non-ST-elevation myocardial infarction (NSTEMI).
- Patients with ST-elevation myocardial infarction (STEMI) when managed with primary or delayed PCI.
Prasugrel tablets have been shown to reduce the rate of a combined endpoint of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke compared to clopidogrel. The difference between treatments was driven predominantly by MI, with no difference on strokes and little difference on CV death
[seeClinical Studies (14)].
Initiate prasugrel tablets treatment as a single 60 mg oral loading dose and then continue at 10 mg orally once daily. Patients taking prasugrel tablets should also take aspirin (75 mg to 325 mg) daily
Prasugrel can be administered with drugs that are inducers or inhibitors of cytochrome P450 enzymes
Prasugrel can be administered with aspirin (75 mg to 325 mg per day), heparin, GPIIb/IIIa inhibitors, statins, digoxin, and drugs that elevate gastric pH, including proton pump inhibitors and H2blockers
Prasugrel is a prodrug and is rapidly metabolized to a pharmacologically active metabolite and inactive metabolites. The active metabolite has an elimination half-life of about 7 hours (range 2 to 15 hours). Healthy subjects, patients with stable atherosclerosis, and patients undergoing PCI show similar pharmacokinetics.
Following oral administration, ≥79% of the dose is absorbed. The absorption and metabolism are rapid, with peak plasma concentrations (Cmax) of the active metabolite occurring approximately 30 minutes after dosing. The active metabolite’s exposure (AUC) increases slightly more than proportionally over the dose range of 5 to 60 mg. Repeated daily doses of 10 mg do not lead to accumulation of the active metabolite. In a study of healthy subjects given a single 15 mg dose, the AUC of the active metabolite was unaffected by a high-fat, high-calorie meal, but Cmaxwas decreased by 49% and Tmaxwas increased from 0.5 to 1.5 hours. Prasugrel can be administered without regard to food. The active metabolite is bound about 98% to human serum albumin.
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel’s active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins. Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites.
Specific Populations
Geriatric Patients
Male and Female Patients
Racial or Ethnic Groups
Smoking
Patients with Renal Impairment
Patients with Hepatic Impairment
Drug Interaction Studies
Potential for Other Drugs to Affect Prasugrel
Inducers of Cytochromes P450
Drugs that Elevate Gastric pH
Statins
Heparin
Aspirin
Warfarin
Potential for Prasugrel to Affect Other Drugs
In vitro
Drugs Metabolized by CYP2B6 -
Effect on Digoxin
Morphine
Prasugrel is a prodrug and is rapidly metabolized to a pharmacologically active metabolite and inactive metabolites. The active metabolite has an elimination half-life of about 7 hours (range 2 to 15 hours). Healthy subjects, patients with stable atherosclerosis, and patients undergoing PCI show similar pharmacokinetics.
Following oral administration, ≥79% of the dose is absorbed. The absorption and metabolism are rapid, with peak plasma concentrations (Cmax) of the active metabolite occurring approximately 30 minutes after dosing. The active metabolite’s exposure (AUC) increases slightly more than proportionally over the dose range of 5 to 60 mg. Repeated daily doses of 10 mg do not lead to accumulation of the active metabolite. In a study of healthy subjects given a single 15 mg dose, the AUC of the active metabolite was unaffected by a high-fat, high-calorie meal, but Cmaxwas decreased by 49% and Tmaxwas increased from 0.5 to 1.5 hours. Prasugrel can be administered without regard to food. The active metabolite is bound about 98% to human serum albumin.
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel’s active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins. Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites.
Specific Populations
Geriatric Patients
Male and Female Patients
Racial or Ethnic Groups
Smoking
Patients with Renal Impairment
Patients with Hepatic Impairment
Drug Interaction Studies
Potential for Other Drugs to Affect Prasugrel
Inducers of Cytochromes P450
Drugs that Elevate Gastric pH
Statins
Heparin
Aspirin
Warfarin
Potential for Prasugrel to Affect Other Drugs
In vitro
Drugs Metabolized by CYP2B6 -
Effect on Digoxin
Morphine
The clinical evidence for the effectiveness of prasugrel is derived from the TRITON-TIMI 38 (
Patients with UA/NSTEMI presenting within 72 hours of symptom onset were to be randomized after undergoing coronary angiography. Patients with STEMI presenting within 12 hours of symptom onset could be randomized prior to coronary angiography. Patients with STEMI presenting between 12 hours and 14 days of symptom onset were to be randomized after undergoing coronary angiography. Patients underwent PCI, and for both UA/NSTEMI and STEMI patients, the loading dose was to be administered anytime between randomization and 1 hour after the patient left the catheterization lab. If patients with STEMI were treated with thrombolytic therapy, randomization could not occur until at least 24 hours (for tenecteplase, reteplase, or alteplase) or 48 hours (for streptokinase) after the thrombolytic was given.
Patients were randomized to receive prasugrel (60 mg loading dose followed by 10 mg once daily) or clopidogrel (300 mg loading dose followed by 75 mg once daily), with administration and follow-up for a minimum of 6 months (actual median 14.5 months). Patients also received aspirin (75 mg to 325 mg once daily). Other therapies, such as heparin and intravenous glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors, were administered at the discretion of the treating physician. Oral anticoagulants, other platelet inhibitors, and chronic NSAIDs were not allowed.
The primary outcome measure was the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in the UA/NSTEMI population. Success in this group allowed analysis of the same endpoint in the overall ACS and STEMI populations. Nonfatal MIs included both MIs detected solely through analysis of creatine kinase muscle-brain (CK-MB) changes and clinically apparent (investigator-reported) MIs.
The patient population was 92% Caucasian, 26% female, and 39% ≥65 years of age. The median time from symptom onset to study drug administration was 7 hours for patients with STEMI and 30 hours for patients with UA/NSTEMI. Approximately 99% of patients underwent PCI. The study drug was administered after the first coronary guidewire was placed in approximately 75% of patients.
Prasugrel significantly reduced total endpoint events compared to clopidogrel (see Figure 3 and Table 5). The reduction of total endpoint events was driven primarily by a decrease in nonfatal MIs, both those occurring early (through 3 days) and later (after 3 days). Approximately 40% of MIs occurred peri-procedurally and were detected solely by changes in CK-MB. Administration of the clopidogrel loading dose in TRITON-TIMI 38 was delayed relative to the placebo-controlled trials that supported its approval for ACS. Prasugrel produced higher rates of clinically significant bleeding than clopidogrel in TRITON-TIMI 38
The treatment effect of prasugrel was apparent within the first few days, and persisted to the end of the study (see Figure 3). The inset shows results over the first 7 days.
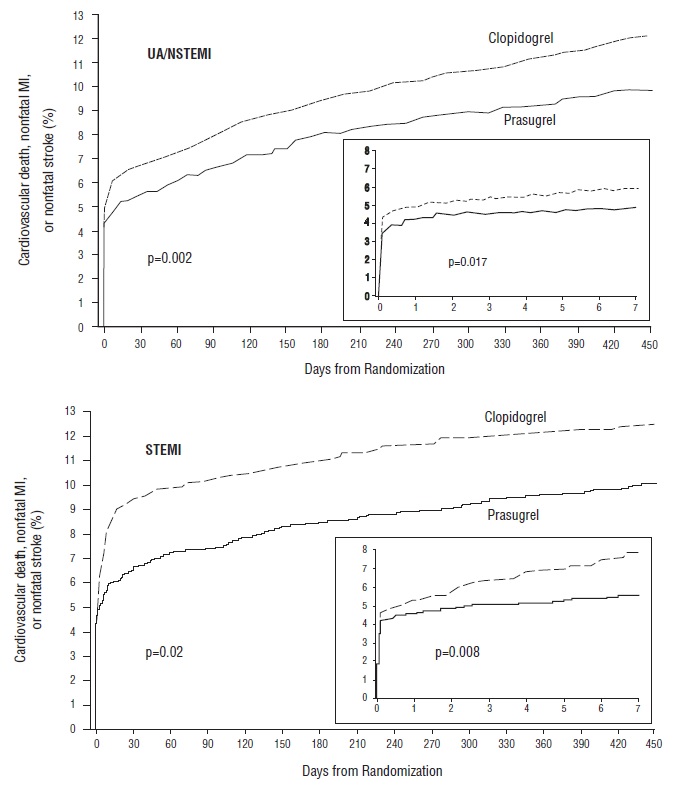
The Kaplan-Meier curves (see Figure 3) show the primary composite endpoint of CV death, nonfatal MI, or nonfatal stroke over time in the UA/NSTEMI and STEMI populations. In both populations, the curves separate within the first few hours. In the UA/NSTEMI population, the curves continue to diverge throughout the 15-month follow-up period. In the STEMI population, the early separation was maintained throughout the 15-month follow-up period, but there was no progressive divergence after the first few weeks.
Prasugrel reduced the occurrence of the primary composite endpoint compared to clopidogrel in both the UA/NSTEMI and STEMI populations (see Table 5). In patients who survived an on-study myocardial infarction, the incidence of subsequent events was also lower in the prasugrel group.
| *RRR = (1-Hazard Ratio) x 100%. Values with a negative relative risk reduction indicate a relative risk increase. | ||||
Patients with events | From Kaplan-Meier analysis | |||
Prasugrel (%) | Clopidogrel (%) | Relative Risk Reduction (%)*(95% CI) | p-value | |
UA/NSTEMI | N=5044 | N=5030 | ||
| CV death, nonfatal MI, or nonfatal stroke | 9.3 | 11.2 | 18.0 (7.3, 27.4) | 0.002 |
| CV death | 1.8 | 1.8 | 2.1 (-30.9, 26.8) | 0.885 |
| Nonfatal MI | 7.1 | 9.2 | 23.9 (12.7, 33.7) | <0.001 |
| Nonfatal Stroke | 0.8 | 0.8 | 2.1 (-51.3, 36.7) | 0.922 |
STEMI | N=1769 | N=1765 | ||
| CV death, nonfatal MI, or nonfatal stroke | 9.8 | 12.2 | 20.7 (3.2, 35.1) | 0.019 |
| CV death | 2.4 | 3.3 | 26.2 (-9.4, 50.3) | 0.129 |
| Nonfatal MI | 6.7 | 8.8 | 25.4 (5.2, 41.2) | 0.016 |
| Nonfatal Stroke | 1.2 | 1.1 | -9.7 (-104.0, 41.0) | 0.77 |
The effect of prasugrel in various subgroups is shown in Figures 4 and 5. Results are generally consistent across pre-specified subgroups, with the exception of patients with a history of TIA or stroke
Figure 4: Subgroup Analyses for Time to First Event of CV Death, MI, or Stroke (HR and 95% CI; TRITON-TIMI 38) – UA/NSTEMI Patients
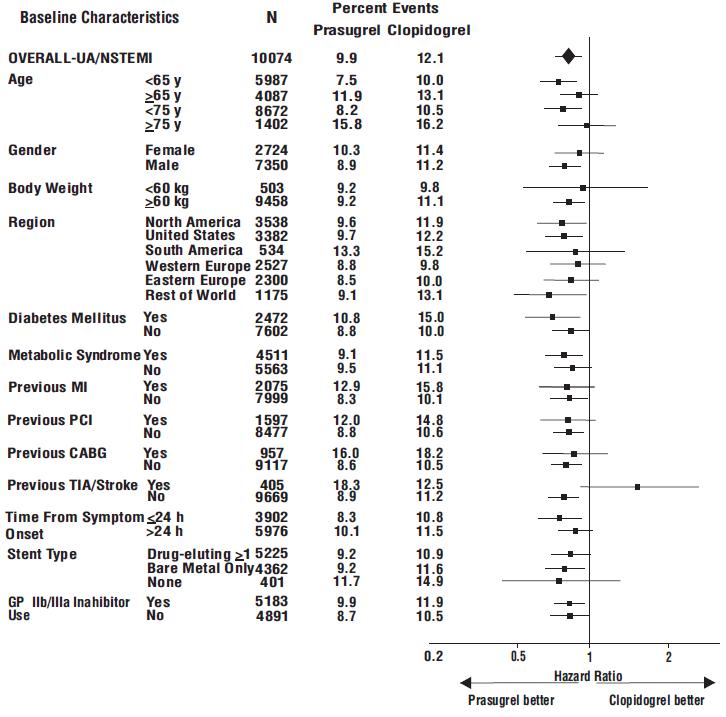
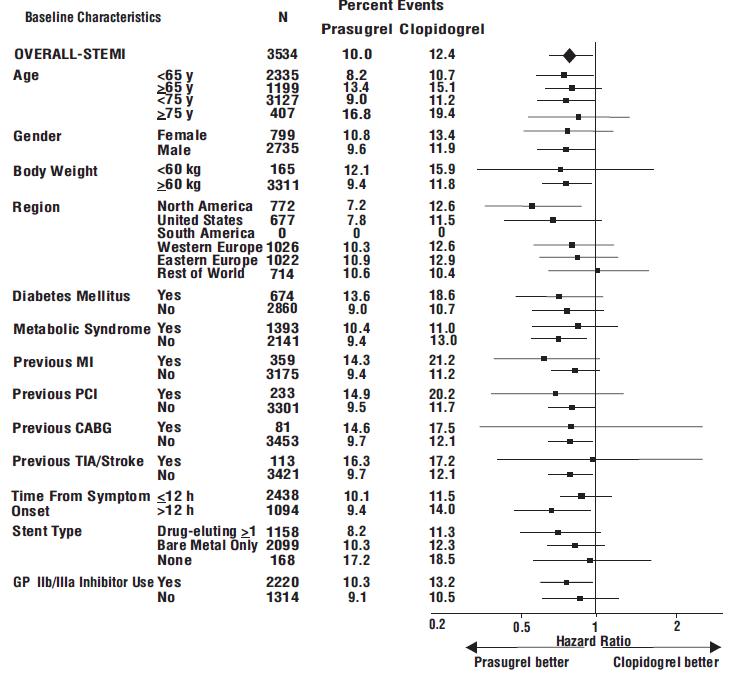
Prasugrel is generally not recommended in patients ≥75 years of age, except in high-risk situations (diabetes mellitus or prior MI) where its effect appears to be greater and its use may be considered. These recommendations are based on subgroup analyses (see Table 6) and must be interpreted with caution, but the data suggest that prasugrel reduces ischemic events in such patients.
Prasugrel | Clopidogrel | Hazard Ratio (95% CI) | |||
N | % with events | N | % with events | ||
Age ≥75 | |||||
| Diabetes –yes | 249 | 14.9 | 234 | 21.8 | 0.64 (0.42, 0.97) |
| Diabetes - no | 652 | 16.4 | 674 | 15.3 | 1.1 (0.83, 1.43) |
Age <75 | |||||
| Diabetes – yes | 1327 | 10.8 | 1336 | 14.8 | 0.72 (0.58, 0.89) |
| Diabetes – no | 4585 | 7.8 | 4551 | 9.5 | 0.82 (0.71, 0.94) |
Age ≥75 | |||||
| Prior MI – yes | 220 | 17.3 | 212 | 22.6 | 0.72 (0.47, 1.09) |
| Prior MI – no | 681 | 15.6 | 696 | 15.2 | 1.05 (0.80, 1.37) |
Age <75 | |||||
| Prior MI – yes | 1006 | 12.2 | 996 | 15.4 | 0.78 (0.62, 0.99) |
| Prior MI – no | 4906 | 7.7 | 4891 | 9.7 | 0.78 (0.68, 0.90) |
There were 50% fewer stent thromboses (95% C.I. 32% to 64%; p<0.001) reported among patients randomized to prasugrel (0.9%) than among patients randomized to clopidogrel (1.8%). The difference manifested early and was maintained through one year of follow-up. Findings were similar with bare metal and drug-eluting stents.
In TRITON-TIMI 38, prasugrel reduced ischemic events (mainly nonfatal MIs) and increased bleeding events



In the clinical trial that established the efficacy and safety of prasugrel tablets, the loading dose of prasugrel tablets was not administered until coronary anatomy was established in UA/NSTEMI patients and in STEMI patients presenting more than 12 hours after symptom onset. In STEMI patients presenting within 12 hours of symptom onset, the loading dose of prasugrel tablets was administered at the time of diagnosis, although most received prasugrel tablets at the time of PCI
The clinical evidence for the effectiveness of prasugrel is derived from the TRITON-TIMI 38 (
Patients with UA/NSTEMI presenting within 72 hours of symptom onset were to be randomized after undergoing coronary angiography. Patients with STEMI presenting within 12 hours of symptom onset could be randomized prior to coronary angiography. Patients with STEMI presenting between 12 hours and 14 days of symptom onset were to be randomized after undergoing coronary angiography. Patients underwent PCI, and for both UA/NSTEMI and STEMI patients, the loading dose was to be administered anytime between randomization and 1 hour after the patient left the catheterization lab. If patients with STEMI were treated with thrombolytic therapy, randomization could not occur until at least 24 hours (for tenecteplase, reteplase, or alteplase) or 48 hours (for streptokinase) after the thrombolytic was given.
Patients were randomized to receive prasugrel (60 mg loading dose followed by 10 mg once daily) or clopidogrel (300 mg loading dose followed by 75 mg once daily), with administration and follow-up for a minimum of 6 months (actual median 14.5 months). Patients also received aspirin (75 mg to 325 mg once daily). Other therapies, such as heparin and intravenous glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors, were administered at the discretion of the treating physician. Oral anticoagulants, other platelet inhibitors, and chronic NSAIDs were not allowed.
The primary outcome measure was the composite of cardiovascular death, nonfatal MI, or nonfatal stroke in the UA/NSTEMI population. Success in this group allowed analysis of the same endpoint in the overall ACS and STEMI populations. Nonfatal MIs included both MIs detected solely through analysis of creatine kinase muscle-brain (CK-MB) changes and clinically apparent (investigator-reported) MIs.
The patient population was 92% Caucasian, 26% female, and 39% ≥65 years of age. The median time from symptom onset to study drug administration was 7 hours for patients with STEMI and 30 hours for patients with UA/NSTEMI. Approximately 99% of patients underwent PCI. The study drug was administered after the first coronary guidewire was placed in approximately 75% of patients.
Prasugrel significantly reduced total endpoint events compared to clopidogrel (see Figure 3 and Table 5). The reduction of total endpoint events was driven primarily by a decrease in nonfatal MIs, both those occurring early (through 3 days) and later (after 3 days). Approximately 40% of MIs occurred peri-procedurally and were detected solely by changes in CK-MB. Administration of the clopidogrel loading dose in TRITON-TIMI 38 was delayed relative to the placebo-controlled trials that supported its approval for ACS. Prasugrel produced higher rates of clinically significant bleeding than clopidogrel in TRITON-TIMI 38
The treatment effect of prasugrel was apparent within the first few days, and persisted to the end of the study (see Figure 3). The inset shows results over the first 7 days.

The Kaplan-Meier curves (see Figure 3) show the primary composite endpoint of CV death, nonfatal MI, or nonfatal stroke over time in the UA/NSTEMI and STEMI populations. In both populations, the curves separate within the first few hours. In the UA/NSTEMI population, the curves continue to diverge throughout the 15-month follow-up period. In the STEMI population, the early separation was maintained throughout the 15-month follow-up period, but there was no progressive divergence after the first few weeks.
Prasugrel reduced the occurrence of the primary composite endpoint compared to clopidogrel in both the UA/NSTEMI and STEMI populations (see Table 5). In patients who survived an on-study myocardial infarction, the incidence of subsequent events was also lower in the prasugrel group.
| *RRR = (1-Hazard Ratio) x 100%. Values with a negative relative risk reduction indicate a relative risk increase. | ||||
Patients with events | From Kaplan-Meier analysis | |||
Prasugrel (%) | Clopidogrel (%) | Relative Risk Reduction (%)*(95% CI) | p-value | |
UA/NSTEMI | N=5044 | N=5030 | ||
| CV death, nonfatal MI, or nonfatal stroke | 9.3 | 11.2 | 18.0 (7.3, 27.4) | 0.002 |
| CV death | 1.8 | 1.8 | 2.1 (-30.9, 26.8) | 0.885 |
| Nonfatal MI | 7.1 | 9.2 | 23.9 (12.7, 33.7) | <0.001 |
| Nonfatal Stroke | 0.8 | 0.8 | 2.1 (-51.3, 36.7) | 0.922 |
STEMI | N=1769 | N=1765 | ||
| CV death, nonfatal MI, or nonfatal stroke | 9.8 | 12.2 | 20.7 (3.2, 35.1) | 0.019 |
| CV death | 2.4 | 3.3 | 26.2 (-9.4, 50.3) | 0.129 |
| Nonfatal MI | 6.7 | 8.8 | 25.4 (5.2, 41.2) | 0.016 |
| Nonfatal Stroke | 1.2 | 1.1 | -9.7 (-104.0, 41.0) | 0.77 |
The effect of prasugrel in various subgroups is shown in Figures 4 and 5. Results are generally consistent across pre-specified subgroups, with the exception of patients with a history of TIA or stroke
Figure 4: Subgroup Analyses for Time to First Event of CV Death, MI, or Stroke (HR and 95% CI; TRITON-TIMI 38) – UA/NSTEMI Patients


Prasugrel is generally not recommended in patients ≥75 years of age, except in high-risk situations (diabetes mellitus or prior MI) where its effect appears to be greater and its use may be considered. These recommendations are based on subgroup analyses (see Table 6) and must be interpreted with caution, but the data suggest that prasugrel reduces ischemic events in such patients.
Prasugrel | Clopidogrel | Hazard Ratio (95% CI) | |||
N | % with events | N | % with events | ||
Age ≥75 | |||||
| Diabetes –yes | 249 | 14.9 | 234 | 21.8 | 0.64 (0.42, 0.97) |
| Diabetes - no | 652 | 16.4 | 674 | 15.3 | 1.1 (0.83, 1.43) |
Age <75 | |||||
| Diabetes – yes | 1327 | 10.8 | 1336 | 14.8 | 0.72 (0.58, 0.89) |
| Diabetes – no | 4585 | 7.8 | 4551 | 9.5 | 0.82 (0.71, 0.94) |
Age ≥75 | |||||
| Prior MI – yes | 220 | 17.3 | 212 | 22.6 | 0.72 (0.47, 1.09) |
| Prior MI – no | 681 | 15.6 | 696 | 15.2 | 1.05 (0.80, 1.37) |
Age <75 | |||||
| Prior MI – yes | 1006 | 12.2 | 996 | 15.4 | 0.78 (0.62, 0.99) |
| Prior MI – no | 4906 | 7.7 | 4891 | 9.7 | 0.78 (0.68, 0.90) |
There were 50% fewer stent thromboses (95% C.I. 32% to 64%; p<0.001) reported among patients randomized to prasugrel (0.9%) than among patients randomized to clopidogrel (1.8%). The difference manifested early and was maintained through one year of follow-up. Findings were similar with bare metal and drug-eluting stents.
In TRITON-TIMI 38, prasugrel reduced ischemic events (mainly nonfatal MIs) and increased bleeding events



Although it is generally recommended that antiplatelet therapy be administered promptly in the management of ACS because many cardiovascular events occur within hours of initial presentation, in a trial of 4033 NSTEMI patients, no clear benefit was observed when prasugrel tablets loading dose was administered prior to diagnostic coronary angiography compared to at the time of PCI; however, risk of bleeding was increased with early administration in patients undergoing PCI or early CABG.
Compared to patients weighing ≥60 kg, patients weighing <60 kg have an increased exposure to the active metabolite of prasugrel and an increased risk of bleeding on a 10 mg once daily maintenance dose. Consider lowering the maintenance dose to 5 mg in patients <60 kg. The effectiveness and safety of the 5 mg dose have not been prospectively studied
Thienopyridines, including prasugrel, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL), bleeding events were more common on prasugrel than on clopidogrel
Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events

Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding.
Do not use prasugrel in patients with active bleeding, prior TIA or stroke
Other risk factors for bleeding are:
- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Adverse Reactions (6.1), Use in Specific Populations (8.5), Clinical Pharmacology (12.3), and Clinical Studies (14)].
- CABG or other surgical procedure[see Warnings and Precautions (5.2)].
- Body weight <60 kg. Consider a lower (5 mg) maintenance dose[see Dosage and Administration (2), Adverse Reactions (6.1), and Use in Specific Populations (8.6)].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment)[see Adverse Reactions (6.1)and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs], and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38[see Drug Interactions (7.1, 7.2, 7.4) and Clinical Studies (14)].
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel’s active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.

Safety in patients with ACS undergoing PCI was evaluated in a clopidogrel-controlled study, TRITON-TIMI 38, in which 6741 patients were treated with prasugrel (60 mg loading dose and 10 mg once daily) for a median of 14.5 months (5802 patients were treated for over 6 months; 4136 patients were treated for more than 1 year). The population treated with prasugrel was 27 to 96 years of age, 25% female, and 92% Caucasian. All patients in the TRITON-TIMI 38 study were to receive aspirin. The dose of clopidogrel in this study was a 300 mg loading dose and 75 mg once daily.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with the rates observed in other clinical trials of another drug and may not reflect the rates observed in practice.
The rate of study drug discontinuation because of adverse reactions was 7.2% for prasugrel and 6.3% for clopidogrel. Bleeding was the most common adverse reaction leading to study drug discontinuation for both drugs (2.5% for prasugrel and 1.4% for clopidogrel).
Bleeding Unrelated to CABG Surgery
| *Patients may be counted in more than one row. †See 5.1for definition. | |||
Prasugrel (%) (N=6741) | Clopidogrel (%) (N=6716) | ||
| TIMI Major or Minor bleeding | 4.5 | 3.4 | |
| TIMI Major bleeding† | 2.2 | 1.7 | |
| Life-threatening | 1.3 | 0.8 | |
| Fatal | 0.3 | 0.1 | |
| Symptomatic intracranial hemorrhage (ICH) | 0.3 | 0.3 | |
| Requiring inotropes | 0.3 | 0.1 | |
| Requiring surgical intervention | 0.3 | 0.3 | |
| Requiring transfusion (≥4 units) | 0.7 | 0.5 | |
| TIMI Minor bleeding† | 2.4 | 1.9 | |
Figure 1 demonstrates non-CABG-related TIMI Major or Minor bleeding. The bleeding rate is highest initially, as shown in Figure 1 (inset: Days 0 to 7)
In TRITON-TIMI 38, non-CABG-related TIMI Major or Minor bleeding rates in patients with the risk factors of age ≥75 years and weight <60 kg are shown in Table 2.
| *10 mg prasugrel maintenance dose †75 mg clopidogrel maintenance dose | ||||
Major/Minor | Fatal | |||
Prasugrel*(%) | Clopidogrel†(%) | Prasugrel*(%) | Clopidogrel†(%) | |
| Weight <60 kg (N=308 prasugrel, N=356 clopidogrel) | 10.1 | 6.5 | 0.0 | 0.3 |
| Weight ≥60 kg (N=6373 prasugrel, N=6299 clopidogrel) | 4.2 | 3.3 | 0.3 | 0.1 |
| Age <75 years (N=5850 prasugrel, N=5822 clopidogrel) | 3.8 | 2.9 | 0.2 | 0.1 |
| Age ≥75 years (N=891 prasugrel, N=894 clopidogrel) | 9.0 | 6.9 | 1.0 | 0.1 |
| *Patients may be counted in more than one row. | ||
Prasugrel (%) (N=213) | Clopidogrel (%) (N=224) | |
| TIMI Major or Minor bleeding | 14.1 | 4.5 |
| TIMI Major bleeding | 11.3 | 3.6 |
| Fatal | 0.9 | 0 |
| Reoperation | 3.8 | 0.5 |
| Transfusion of ≥5 units | 6.6 | 2.2 |
| Intracranial hemorrhage | 0 | 0 |
| TIMI Minor bleeding | 2.8 | 0.9 |
During TRITON-TIMI 38, newly diagnosed malignancies were reported in 1.6% and 1.2% of patients treated with prasugrel and clopidogrel, respectively. The sites contributing to the differences were primarily colon and lung. In another Phase 3 clinical study of ACS patients not undergoing PCI, in which data for malignancies were prospectively collected, newly diagnosed malignancies were reported in 1.8% and 1.7% of patients treated with prasugrel and clopidogrel, respectively. The site of malignancies was balanced between treatment groups except for colorectal malignancies. The rates of colorectal malignancies were 0.3% prasugrel, 0.1% clopidogrel and most were detected during investigation of GI bleed or anemia. It is unclear if these observations are causally related, are the result of increased detection because of bleeding, or are random occurrences.
In TRITON-TIMI 38, common and other important nonhemorrhagic adverse events were, for prasugrel and clopidogrel, respectively: severe thrombocytopenia (0.06%, 0.04%), anemia (2.2%, 2.0%), abnormal hepatic function (0.22%, 0.27%), allergic reactions (0.36%, 0.36%), and angioedema (0.06%, 0.04%). Table 4 summarizes the adverse events reported by at least 2.5% of patients.
Prasugrel (%) (N=6741) | Clopidogrel (%) (N=6716) | |
| Hypertension | 7.5 | 7.1 |
| Hypercholesterolemia/Hyperlipidemia | 7.0 | 7.4 |
| Headache | 5.5 | 5.3 |
| Back pain | 5.0 | 4.5 |
| Dyspnea | 4.9 | 4.5 |
| Nausea | 4.6 | 4.3 |
| Dizziness | 4.1 | 4.6 |
| Cough | 3.9 | 4.1 |
| Hypotension | 3.9 | 3.8 |
| Fatigue | 3.7 | 4.8 |
| Noncardiac chest pain | 3.1 | 3.5 |
| Atrial fibrillation | 2.9 | 3.1 |
| Bradycardia | 2.9 | 2.4 |
| Leukopenia (<4 x 109WBC*/L) | 2.8 | 3.5 |
| Rash | 2.8 | 2.4 |
| Pyrexia | 2.7 | 2.2 |
| Peripheral edema | 2.7 | 3.0 |
| Pain in extremity | 2.6 | 2.6 |
| Diarrhea | 2.3 | 2.6 |
*WBC = white blood cell
Prasugrel is a prodrug and is rapidly metabolized to a pharmacologically active metabolite and inactive metabolites. The active metabolite has an elimination half-life of about 7 hours (range 2 to 15 hours). Healthy subjects, patients with stable atherosclerosis, and patients undergoing PCI show similar pharmacokinetics.
Following oral administration, ≥79% of the dose is absorbed. The absorption and metabolism are rapid, with peak plasma concentrations (Cmax) of the active metabolite occurring approximately 30 minutes after dosing. The active metabolite’s exposure (AUC) increases slightly more than proportionally over the dose range of 5 to 60 mg. Repeated daily doses of 10 mg do not lead to accumulation of the active metabolite. In a study of healthy subjects given a single 15 mg dose, the AUC of the active metabolite was unaffected by a high-fat, high-calorie meal, but Cmaxwas decreased by 49% and Tmaxwas increased from 0.5 to 1.5 hours. Prasugrel can be administered without regard to food. The active metabolite is bound about 98% to human serum albumin.
Prasugrel is not detected in plasma following oral administration. It is rapidly hydrolyzed in the intestine to a thiolactone, which is then converted to the active metabolite by a single step, primarily by CYP3A4 and CYP2B6 and to a lesser extent by CYP2C9 and CYP2C19. The estimates of apparent volume of distribution of prasugrel’s active metabolite ranged from 44 to 68 L and the estimates of apparent clearance ranged from 112 to 166 L/hr in healthy subjects and patients with stable atherosclerosis. The active metabolite is metabolized to two inactive compounds by S-methylation or conjugation with cysteine. The major inactive metabolites are highly bound to human plasma proteins. Approximately 68% of the prasugrel dose is excreted in the urine and 27% in the feces as inactive metabolites.
Specific Populations
Geriatric Patients
Male and Female Patients
Racial or Ethnic Groups
Smoking
Patients with Renal Impairment
Patients with Hepatic Impairment
Drug Interaction Studies
Potential for Other Drugs to Affect Prasugrel
Inducers of Cytochromes P450
Drugs that Elevate Gastric pH
Statins
Heparin
Aspirin
Warfarin
Potential for Prasugrel to Affect Other Drugs
In vitro
Drugs Metabolized by CYP2B6 -
Effect on Digoxin
Morphine
Prasugrel Tablets USP, 5 mg are available as yellow, elongated hexagonal, film-coated, non-scored tablets debossed with ‘I’ on one side and ‘23’ on the other side.
Prasugrel Tablets USP, 10 mg are available as beige, elongated hexagonal, film-coated, non-scored tablets debossed with ‘I’ on one side and ‘24’ on the other side.
There are no data with prasugrel use in pregnant women to inform a drug-associated risk. No structural malformations were observed in animal reproductive and developmental toxicology studies when rats and rabbits were administered prasugrel during organogenesis at doses of up to 30 times the recommended therapeutic exposures in humans
- Prasugrel can cause significant, sometimes fatal, bleeding[see Warnings and Precautions (5.1, 5.2)and Adverse Reactions (6.1)].
- Do not use prasugrel in patients with active pathological bleeding or a history of transient ischemic attack (TIA) or stroke[see Contraindications (4.1, 4.2)].
- In patients ≥75 years of age, prasugrel is generally not recommended, because of the increased risk of fatal and intracranial bleeding and uncertain benefit, except in high-risk situations (patients with diabetes or a history of prior myocardial infarction [MI]) where its effect appears to be greater and its use may be considered[see Use in Specific Populations (8.5)].
- Do not start prasugrel in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue prasugrel at least 7 days prior to any surgery[see Warnings and Precautions (5.2)].
- Additional risk factors for bleeding include: body weight <60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding (e.g., warfarin, heparin, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs])[see Warnings and Precautions (5.1)].
- Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, percutaneous coronary intervention (PCI), CABG, or other surgical procedures in the setting of prasugrel[see Warnings and Precautions (5.1)].
- If possible, manage bleeding without discontinuing prasugrel. Discontinuing prasugrel, particularly in the first few weeks after acute coronary syndrome, increases the risk of subsequent cardiovascular (CV) events[see Warnings and Precautions (5.3)].
- Prasugrel can cause significant, sometimes fatal, bleeding (5.1, 5.2, 6.1).
- Do not use prasugrel in patients with active pathological bleeding or a history of transient ischemic attack or stroke (4.1, 4.2).
- In patients≥75 years of age, prasugrel is generally not recommended, except in high-risk patients (diabetes or priormyocardial infarction [MI]), where its use may be considered(8.5).
- Do not start prasugrel in patients likely to undergo urgent coronary artery bypass graft surgery (CABG). When possible, discontinue prasugrel at least 7 days prior to any surgery(5.2).
- Additional risk factors for bleeding include: body weight <60 kg, propensity to bleed, concomitant use of medications that increase the risk of bleeding(5.1).
- Suspect bleeding in any patient who is hypotensive and has recently undergoneinvasive or surgical procedures(5.1).
- If possible, manage bleeding without discontinuing prasugrel. Stopping prasugrel increases the risk of subsequent cardiovascular events(5.3).
Thienopyridines, including prasugrel, increase the risk of bleeding. With the dosing regimens used in TRITON-TIMI 38, TIMI (Thrombolysis in Myocardial Infarction) Major (clinically overt bleeding associated with a fall in hemoglobin ≥5 g/dL, or intracranial hemorrhage) and TIMI Minor (overt bleeding associated with a fall in hemoglobin of ≥3 g/dL but <5 g/dL), bleeding events were more common on prasugrel than on clopidogrel
Figure 1: Non-CABG-Related TIMI Major or Minor Bleeding Events

Suspect bleeding in any patient who is hypotensive and has recently undergone coronary angiography, PCI, CABG, or other surgical procedures even if the patient does not have overt signs of bleeding.
Do not use prasugrel in patients with active bleeding, prior TIA or stroke
Other risk factors for bleeding are:
- Age ≥75 years. Because of the risk of bleeding (including fatal bleeding) and uncertain effectiveness in patients ≥75 years of age, use of prasugrel is generally not recommended in these patients, except in high-risk situations (patients with diabetes or history of myocardial infarction) where its effect appears to be greater and its use may be considered[see Adverse Reactions (6.1), Use in Specific Populations (8.5), Clinical Pharmacology (12.3), and Clinical Studies (14)].
- CABG or other surgical procedure[see Warnings and Precautions (5.2)].
- Body weight <60 kg. Consider a lower (5 mg) maintenance dose[see Dosage and Administration (2), Adverse Reactions (6.1), and Use in Specific Populations (8.6)].
- Propensity to bleed (e.g., recent trauma, recent surgery, recent or recurrent gastrointestinal (GI) bleeding, active peptic ulcer disease, severe hepatic impairment, or moderate to severe renal impairment)[see Adverse Reactions (6.1)and Use in Specific Populations (8.7, 8.8)].
- Medications that increase the risk of bleeding (e.g., oral anticoagulants, chronic use of nonsteroidal anti-inflammatory drugs [NSAIDs], and fibrinolytic agents). Aspirin and heparin were commonly used in TRITON-TIMI 38[see Drug Interactions (7.1, 7.2, 7.4) and Clinical Studies (14)].
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of prasugrel’s active metabolite is short relative to the lifetime of the platelet, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 6 hours of the loading dose or 4 hours of the maintenance dose may be less effective.

Discontinue thienopyridines, including prasugrel, for active bleeding, elective surgery, stroke, or TIA. The optimal duration of thienopyridine therapy is unknown. In patients who are managed with PCI and stent placement, premature discontinuation of any antiplatelet medication, including thienopyridines, conveys an increased risk of stent thrombosis, myocardial infarction, and death. Patients who require premature discontinuation of a thienopyridine will be at increased risk for cardiac events. Lapses in therapy should be avoided, and if thienopyridines must be temporarily discontinued because of an adverse event(s), they should be restarted as soon as possible
The background risk of major birth defects and miscarriage for the indicated population is unknown. The background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
In embryo-fetal developmental toxicology studies, pregnant rats and rabbits received prasugrel at maternally toxic oral doses equivalent to more than 40 times the human exposure. A slight decrease in fetal body weight was observed, but there were no structural malformations in either species. In prenatal and postnatal rat studies, maternal treatment with prasugrel had no effect on the behavioral or reproductive development of the offspring at doses greater than 150 times the human exposure.
- Active pathological bleeding
4.1 Active BleedingPrasugrel tablets are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage (ICH)
[see Warnings and Precautions (5.1)and Adverse Reactions (6.1)]. - Prior transient ischemic attack or stroke
4.2 Prior Transient Ischemic Attack or StrokePrasugrel tablets are contraindicated in patients with a history of prior transient ischemic attack (TIA) or stroke. In TRITON-TIMI 38 (
TRial to AssessImprovement inTherapeutic Outcomes byOptimizing Platelet InhibitioNwith Prasugrel), patients with a history of TIA or ischemic stroke (>3 months prior to enrollment) had a higher rate of stroke on prasugrel tablets (6.5%; of which 4.2% were thrombotic stroke and 2.3% were intracranial hemorrhage [ICH]) than on clopidogrel (1.2%; all thrombotic). In patients without such a history, the incidence of stroke was 0.9% (0.2% ICH) and 1.0% (0.3% ICH) with prasugrel tablets and clopidogrel, respectively. Patients with a history of ischemic stroke within 3 months of screening and patients with a history of hemorrhagic stroke at any time were excluded from TRITON-TIMI 38. Patients who experience a stroke or TIA while on prasugrel tablets generally should have therapy discontinued[see Adverse Reactions (6.1)and Clinical Studies (14)]. - Hypersensitivity to prasugrel or any component of the product
4.3 HypersensitivityPrasugrel tablets are contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to prasugrel or any component of the product
[see Adverse Reactions (6.2)].