Prazosin Hydrochloride
Prazosin Hydrochloride Prescribing Information
Prazosin Hydrochloride Capsules, USP is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including this drug.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Prazosin Hydrochloride Capsules, USP can be used alone or in combination with other antihypertensive drugs such as diuretics or beta-adrenergic blocking agents.
The dose of prazosin hydrochloride capsules should be adjusted according to the patient’s individual blood pressure response. The following is a guide to its administration:
1 mg two or three times a day (see WARNINGS).
Dosage may be slowly increased to a total daily dose of 20 mg given in divided doses. The therapeutic dosages most commonly employed have ranged from 6 mg to 15 mg daily given in divided doses. Doses higher than 20 mg usually do not increase efficacy, however a few patients may benefit from further increases up to a daily dose of 40 mg given in divided doses. After initial titration some patients can be maintained adequately on a twice daily dosage regimen.
When adding a diuretic or other antihypertensive agent, the dose of prazosin hydrochloride capsules should be reduced to 1 mg or 2 mg three times a day and retitration then carried out.
Concomitant administration of prazosin hydrochloride capsules with a PDE-5 inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension; therefore, PDE-5 inhibitor therapy should be initiated at the lowest dose in patients taking prazosin hydrochloride capsules.
Prazosin Hydrochloride Capsules are contraindicated in patients with known sensitivity to quinazolines, prazosin, or any of the inert ingredients.
Clinical trials were conducted on more than 900 patients. During these trials and subsequent marketing experience, the most frequent reactions associated with prazosin hydrochloride therapy are: dizziness 10.3%, headache 7.8%, drowsiness 7.6%, lack of energy 6.9%, weakness 6.5%, palpitations 5.3%, and nausea 4.9%. In most instances, side effects have disappeared with continued therapy or have been tolerated with no decrease in dose of drug.
Less frequent adverse reactions which are reported to occur in 1% to 4% of patients are:
In addition, fewer than 1% of patients have reported the following (in some instances, exact causal relationships have not been established):
Single reports of pigmentary mottling and serous retinopathy, and a few reports of cataract development or disappearance have been reported. In these instances, the exact causal relationship has not been established because the baseline observations were frequently inadequate.
In more specific slit-lamp and funduscopic studies, which included adequate baseline examinations, no drug-related abnormal ophthalmological findings have been reported.
Literature reports exist associating prazosin hydrochloride therapy with a worsening of preexisting narcolepsy. A causal relationship is uncertain in these cases.
In post-marketing experience, the following adverse events have been reported:
Prazosin hydrochloride has been administered without any adverse drug interaction in limited clinical experience to date with the following: (1) cardiac glycosides-digitalis and digoxin; (2) hypoglycemics-insulin, chlorpropamide, phenformin, tolazamide, and tolbutamide; (3) tranquilizers and sedatives-chlordiazepoxide, diazepam, and phenobarbital; (4) antigout-allopurinol, colchicine, and probenecid; (5) antiarrhythmics-procainamide, propranolol (see WARNINGS however), and quinidine; and (6) analgesics, antipyretics and anti-inflammatories-propoxyphene, aspirin, indomethacin, and phenylbutazone.
Addition of a diuretic or other antihypertensive agent to prazosin hydrochloride has been shown to cause an additive hypotensive effect. This effect can be minimized by reducing the prazosin hydrochloride dose to 1 mg to 2 mg three times a day, by introducing additional antihypertensive drugs cautiously, and then by retitrating prazosin hydrochloride based on clinical response.
Concomitant administration of prazosin hydrochloride with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension (see DOSAGE AND ADMINISTRATION).
Prazosin hydrochloride, USP a quinazoline derivative, is the first of a new chemical class of antihypertensives. It is the hydrochloride salt of 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl) piperazine and its structural formula is: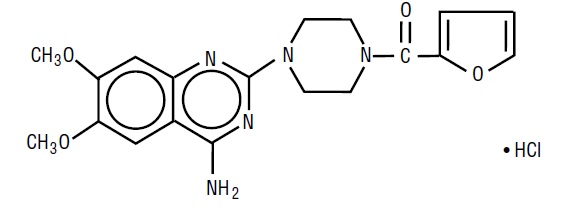
Molecular formula C19H21N5O4•HCl
It is a white to tan powder, slightly soluble in water, practically insoluble in chloroform and acetone and has a molecular weight of 419.87. Each capsule, for oral administration, contains prazosin hydrochloride, USP equivalent (as the polyhydrate) to 1 mg, 2 mg or 5 mg of prazosin.
Inert ingredients in the formulations are: colloidal silicon dioxide, lactose monohydrate, lactose anhydrous, magnesium stearate and microcrystalline cellulose. The empty hard gelatin capsules contain black iron oxide, gelatin, red iron oxide, titanium dioxide and yellow iron oxide. In addition, the 1 mg empty gelatin capsules contain D&C Yellow No. 10 and FD&C Green No. 3; the 2 mg empty gelatin capsules contain D&C Red No. 28, D&C Yellow No. 10, FD&C Blue No. 1 and FD&C Red No. 40; and the 5 mg empty gelatin capsules contain FD&C Blue No. 1.
The capsules shells are imprinted in edible ink which contains concentrated ammonium solution, potassium hydroxide, propylene glycol, shellac and titanium dioxide.