Prednisone
Prednisone Prescribing Information
For palliative management of: leukemias and lymphomas in adults, acute leukemia of childhood.
Gastrointestinal diseases
In addition to the above indications, prednisone tablets are indicated for systemic dermatomyositis (polymyositis).
Hormone therapy is an adjunct to, and not a replacement for, conventional therapy.
Dosage should be decreased or discontinued gradually when the drug has been administered for more than a few days.
The severity, prognosis, expected duration of the disease, and the reaction of the patient to medication are primary factors in determining dosage. If a period of spontaneous remission occurs in a chronic condition, treatment should be discontinued.
Blood pressure, body weight, routine laboratory studies, including two-hour postprandial blood glucose and serum potassium, and a chest X-ray should be obtained at regular intervals during prolonged therapy. Upper GI X-rays are desirable in patients with known or suspected peptic ulcer disease
The initial dosage of prednisone may vary from 5 mg to 60 mg per day, depending on the specific disease entity being treated. In situations of less severity lower doses will generally suffice, while in selected patients higher initial doses may be required. The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, prednisone should be discontinued, and the patient transferred to other appropriate therapy.
Glucocorticoids are adrenocortical steroids, both naturally occurring and synthetic, that are readily absorbed from the gastrointestinal tract. The formula for prednisone is C21H26O5. Chemically, it is 17,21-dihydroxypregna-1,4-diene-3,11,20-trione and has the following structure.:
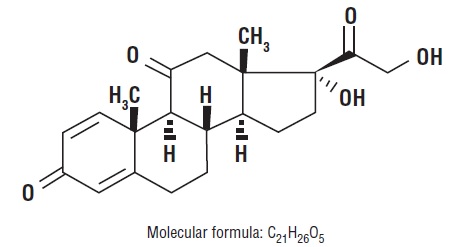
Prednisone USP is a white to partially white, odorless, crystalline powder and has a molecular weight of 358.43. It melts at about 230°C with some decomposition.
Prednisone USP is very slightly soluble in water; slightly soluble in alcohol, chloroform, dioxane, and methanol. Prednisone tablets USP contain 1 mg prednisone, USP.
The inactive ingredients for prednisone tablets USP include: D&C yellow No.10 aluminum lake, FD&C yellow # 6 aluminum lake, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch (maize), and sodium starch glycolate.
Meets USP Dissolution Test 2.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, such as prednisone, are primarily used for their potent anti-inflammatory effects in disorders of many organ systems. Glucocorticoids, such as prednisone, cause profound and varied metabolic effects. In addition, they modify the body's immune response to diverse stimuli.