Primidone
Primidone Prescribing Information
Primidone tablets, used alone or concomitantly with other anticonvulsants, are indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy.
Patients 8 years of age and older who have received no previous treatment may be started on primidone tablets according to the following regimen using either 50 mg or scored 250 mg primidone tablets:
Days 1 to 3: 100 to 125 mg at bedtime
Days 4 to 6: 100 to 125 mg b.i.d.
Days 7 to 9: 100 to 125 mg t.i.d.
Day 10 to maintenance: 250 mg t.i.d.
For most adults and children 8 years of age and over, the usual maintenance dosage is three to four 250 mg primidone tablets in divided doses (250 mg t.i.d. or q.i.d.). If required, an increase to five or six 250 mg tablets daily may be made but daily doses should not exceed 500 mg q.i.d.
| INITIAL:ADULTS AND CHILDREN OVER 8 | ||||||
|---|---|---|---|---|---|---|
KEY:• = 50 mg tablet; ● = 250 mg tablet | ||||||
| DAY | 1 | 2 | 3 | 4 | 5 | 6 |
| AM | •• | •• | •• | |||
| NOON | ||||||
| PM | •• | •• | •• | •• | •• | •• |
| DAY | 7 | 8 | 9 | 10 | 11 | 12 |
| AM | •• | •• | •• | ● | Adjust to Maintenance | |
| NOON | •• | •• | •• | ● | ||
| PM | •• | •• | •• | ● | ||
Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations of primidone tablets may be necessary for optimal dosage adjustment. The clinically effective serum level for primidone tablets is between 5 to 12 μg/mL.
Primidone tablet is contraindicated in:
1) patients with porphyria and
2) patients who are hypersensitive to phenobarbital (see
Primidone raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known.
Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, PEMA potentiates the anticonvulsant activity of phenobarbital in experimental animals.
Primidone raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known.
Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, PEMA potentiates the anticonvulsant activity of phenobarbital in experimental animals.
The most frequently occurring early side effects are ataxia and vertigo. These tend to disappear with continued therapy, or with reduction of initial dosage. Occasionally, the following have been reported: nausea, anorexia, vomiting, fatigue, hyperirritability, emotional disturbances, sexual impotency, diplopia, nystagmus, drowsiness and morbilliform skin eruptions. Granulocytopenia, agranulocytosis, and redcell hypoplasia and aplasia, have been reported rarely. These and, occasionally, other persistent or severe side effects may necessitate withdrawal of the drug. Megaloblastic anemia may occur as a rare idiosyncrasy to primidone and to other anticonvulsants. The anemia responds to folic acid without necessity of discontinuing medication.
Structural formula:
C12H14N2O2
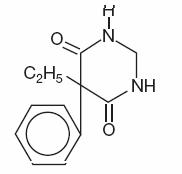 M.W. 218.25
M.W. 218.25Primidone is a white crystalline powder, M.P. 279-284°C. It is very slightly soluble in methanol and methylene dichloride, slightly soluble in alcohol and insoluble in water. It possesses no acidic properties, in contrast to its barbiturate analog.
Primidone tablets 50 mg and 250 mg contain the following inactive ingredients: lactose monohydrate, magnesium stearate, methyl cellulose, purified water, sodium lauryl sulphate, sodium starch glycolate and talc.
Primidone tablets 250 mg also contain yellow iron oxide.
Primidone raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known.
Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, PEMA potentiates the anticonvulsant activity of phenobarbital in experimental animals.