Prismasol
(Magnesium Chloride, Dextrose Anhydrous, Lactic Acid, Sodium Chloride, Sodium Bicarbonate, And Potassium Chloride)Prismasol Prescribing Information
PRISMASOL and PHOXILLUM solutions are indicated in pediatric and adult patients for use as a replacement solution in Continuous Renal Replacement Therapy (CRRT) to replace plasma volume removed by ultrafiltration and to correct electrolyte and acid-base imbalances. They may also be used in case of drug poisoning when CRRT is used to remove dialyzable substances.
- Therapy must be individualized based on the patient's clinical condition, fluid, electrolyte, acid-base and glucose balance ()
2.2 Dosing ConsiderationsPRISMASOL replacement solutions contain 4 different combinations of active ingredients (7 different products with varying ingredient amounts). PHOXILLUM replacement solutions contain 2 different combinations of active ingredients (2 different products with varying ingredient amounts). PRISMASOL and PHOXILLUM are supplied in a two-compartment bag that must be mixed immediately prior to use
[see Dosage and Administration (2.3)]:- Small compartment A (250 mL) containing an electrolyte solution, and
- Large compartment B (4750 mL) containing the buffer solution.
See Table 1for the concentrations of the active ingredients (after mixing) in these 9 different replacement solutions (total volume is 5 Liters).
Table 1: Concentrations of Active Ingredients in the 7 PRISMASOL and 2 PHOXILLUM Replacement Solutions after Mixing Ca2+mEq/LHCO3-mEq/LK+mEq/LMg2+mEq/LNa+mEq/LHPO42-mmol/LCl-mEq/LLactatemEq/LDextrosemg/dLOsmolaritymOsm/LCa2+= calcium, HCO3-= bicarbonate, K+= potassium, Mg2+= magnesium, Na+= sodium, HPO42-= phosphate, Cl- = chloride; osmolarity is estimated PRISMASOL Replacement SolutionsBGK0/2.5
2.5
32
0
1.5
140
0
109
3
100
292
BGK4/2.5
2.5
32
4
1.5
140
0
113
3
100
300
BGK2/3.5
3.5
32
2
1
140
0
111.5
3
100
296
BGK2/0
0
32
2
1
140
0
108
3
100
291
B22GK4/0
0
22
4
1.5
140
0
120.5
3
100
296
BGK4/0/1.2
0
32
4
1.2
140
0
110.2
3
100
295
BK0/0/1.2
0
32
0
1.2
140
0
106.2
3
0
282
PHOXILLUM Replacement Solutions
BK4/2.5
2.5
32
4
1.5
140
1
114.5
0
0
294
B22K4/0
0
22
4
1.5
140
1
122
0
0
290
The mode of therapy, solute formulation, flow rates, and length of PRISMASOL and PHOXILLUM replacement therapy in CRRT should be established by a physician based on the patient’s clinical condition, blood concentration of phosphate and other electrolytes, acid-base and glucose balance. Administer either PRISMASOL or PHOXILLUM into the extracorporeal circuit:
- Before (pre-dilution) the hemofilter or hemodiafilter,
- After (post-dilution) the hemofilter or hemodiafilter, or
- Before and after the hemofilter or hemodiafilter.
- Solution must be mixed prior to use ()
2.2 Dosing ConsiderationsPRISMASOL replacement solutions contain 4 different combinations of active ingredients (7 different products with varying ingredient amounts). PHOXILLUM replacement solutions contain 2 different combinations of active ingredients (2 different products with varying ingredient amounts). PRISMASOL and PHOXILLUM are supplied in a two-compartment bag that must be mixed immediately prior to use
[see Dosage and Administration (2.3)]:- Small compartment A (250 mL) containing an electrolyte solution, and
- Large compartment B (4750 mL) containing the buffer solution.
See Table 1for the concentrations of the active ingredients (after mixing) in these 9 different replacement solutions (total volume is 5 Liters).
Table 1: Concentrations of Active Ingredients in the 7 PRISMASOL and 2 PHOXILLUM Replacement Solutions after Mixing Ca2+mEq/LHCO3-mEq/LK+mEq/LMg2+mEq/LNa+mEq/LHPO42-mmol/LCl-mEq/LLactatemEq/LDextrosemg/dLOsmolaritymOsm/LCa2+= calcium, HCO3-= bicarbonate, K+= potassium, Mg2+= magnesium, Na+= sodium, HPO42-= phosphate, Cl- = chloride; osmolarity is estimated PRISMASOL Replacement SolutionsBGK0/2.5
2.5
32
0
1.5
140
0
109
3
100
292
BGK4/2.5
2.5
32
4
1.5
140
0
113
3
100
300
BGK2/3.5
3.5
32
2
1
140
0
111.5
3
100
296
BGK2/0
0
32
2
1
140
0
108
3
100
291
B22GK4/0
0
22
4
1.5
140
0
120.5
3
100
296
BGK4/0/1.2
0
32
4
1.2
140
0
110.2
3
100
295
BK0/0/1.2
0
32
0
1.2
140
0
106.2
3
0
282
PHOXILLUM Replacement Solutions
BK4/2.5
2.5
32
4
1.5
140
1
114.5
0
0
294
B22K4/0
0
22
4
1.5
140
1
122
0
0
290
The mode of therapy, solute formulation, flow rates, and length of PRISMASOL and PHOXILLUM replacement therapy in CRRT should be established by a physician based on the patient’s clinical condition, blood concentration of phosphate and other electrolytes, acid-base and glucose balance. Administer either PRISMASOL or PHOXILLUM into the extracorporeal circuit:
- Before (pre-dilution) the hemofilter or hemodiafilter,
- After (post-dilution) the hemofilter or hemodiafilter, or
- Before and after the hemofilter or hemodiafilter.
- Use only with extracorporeal dialysis equipment appropriate for CRRT ()
2.3 Preparing the SolutionUse only if the overwrap is not damaged, all seals are intact, peel seal is not broken, and the solution is clear.
The solution may be warmed to 37°C/98.6°F prior to removing the overwrap to enhance patient comfort. However, only dry heat should be used. Solutions should not be heated in water or in a microwave oven. After heating, verify that the solution remains clear and contains no particulate matter.
The solutions are supplied in two different two-compartment bags made of polyolefin with a peel seal separating compartment A and B (see Figure 1).
Follow the instructions below when connecting the solution bags for correct use of the access ports.
Instructions for preparing solutions supplied in a two-compartment, polyolefin bag with a peel seal: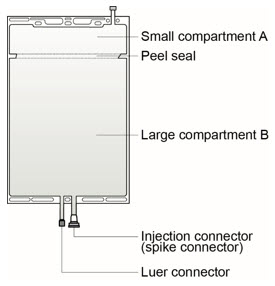

Figure 1 Figure 2
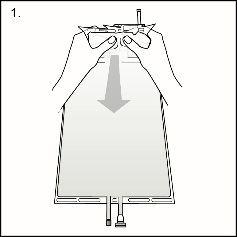 Step 1Immediately before use, remove the overwrap from the bag and mix the solutions in the two different compartments. After removing the overwrap, inspect the bag for leakage by pressing firmly on the bag. Discard the bag if any leakage is detected since sterility cannot be assured. As soon as the overwrap is removed, the reconstitution of compartments A and B should be done and the mixed solution should be used immediately.
Step 1Immediately before use, remove the overwrap from the bag and mix the solutions in the two different compartments. After removing the overwrap, inspect the bag for leakage by pressing firmly on the bag. Discard the bag if any leakage is detected since sterility cannot be assured. As soon as the overwrap is removed, the reconstitution of compartments A and B should be done and the mixed solution should be used immediately.After removal of the overwrap, the solution is stable for 24 hours including the duration of the treatment.
Hold the small compartment with both hands and squeeze it until an opening is created in the peel seal. (See Figure 2beside)
Figure 3
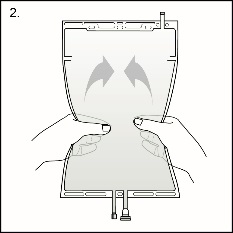 Step 2Squeeze with both hands on the large compartment until the peel seal between the two compartments is entirely open. Shake gently to mix. (See Figure 3beside)
Step 2Squeeze with both hands on the large compartment until the peel seal between the two compartments is entirely open. Shake gently to mix. (See Figure 3beside)The solution is now ready to use and the bag can be hung on the equipment.
Figure 4a
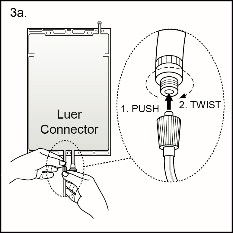 Step 3The replacement line may be connected to the bag through either of the luer connector or the injection connector (spike connector).Step 3aThe luer connector is a needle-less and swabbable connector. Remove the cap with a twist and pull motion, and connect the male luer lock on the replacement line to the female luer receptor on the bag. (See Figure 4a beside)
Step 3The replacement line may be connected to the bag through either of the luer connector or the injection connector (spike connector).Step 3aThe luer connector is a needle-less and swabbable connector. Remove the cap with a twist and pull motion, and connect the male luer lock on the replacement line to the female luer receptor on the bag. (See Figure 4a beside)Ensure that the connection is fully seated and tighten. The connector is now open. Verify that the fluid is flowing freely during use.
When the replacement line is disconnected from the luer connector, the connector will close and the flow of the solution will stop.
Figure 4b
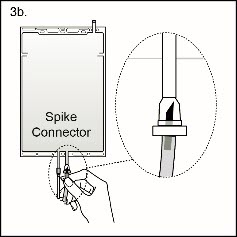 Step 3bIf the injection connector (spike connector) is used, first remove the snap-off cap. Then introduce the replacement line spike through the swabbable rubber septum of the bag connector. (See Figure 4b beside)
Step 3bIf the injection connector (spike connector) is used, first remove the snap-off cap. Then introduce the replacement line spike through the swabbable rubber septum of the bag connector. (See Figure 4b beside)Ensure that the spike is fully inserted and verify that the fluid is flowing freely during use.

Figure 2 
Figure 3 
Figure 4a 
Figure 4b
See
Ca2+ mEq/L | HCO3- mEq/L | K+ mEq/L | Mg2+ mEq/L | Na+ mEq/L | HPO42- mmol/L | Cl- mEq/L | Lactate mEq/L | Dextrose mg/dL | Osmolarity mOsm/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+= calcium, HCO3-= bicarbonate, K+= potassium, Mg2+= magnesium, Na+= sodium, HPO42-= phosphate, Cl- = chloride; osmolarity is estimated | ||||||||||
PRISMASOL Replacement Solutions | ||||||||||
BGK0/2.5 | 2.5 | 32 | 0 | 1.5 | 140 | 0 | 109 | 3 | 100 | 292 |
BGK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 0 | 113 | 3 | 100 | 300 |
BGK2/3.5 | 3.5 | 32 | 2 | 1 | 140 | 0 | 111.5 | 3 | 100 | 296 |
BGK2/0 | 0 | 32 | 2 | 1 | 140 | 0 | 108 | 3 | 100 | 291 |
B22GK4/0 | 0 | 22 | 4 | 1.5 | 140 | 0 | 120.5 | 3 | 100 | 296 |
BGK4/0/1.2 | 0 | 32 | 4 | 1.2 | 140 | 0 | 110.2 | 3 | 100 | 295 |
BK0/0/1.2 | 0 | 32 | 0 | 1.2 | 140 | 0 | 106.2 | 3 | 0 | 282 |
PHOXILLUM Replacement Solutions | ||||||||||
BK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 1 | 114.5 | 0 | 0 | 294 |
B22K4/0 | 0 | 22 | 4 | 1.5 | 140 | 1 | 122 | 0 | 0 | 290 |
PRISMASOL replacement solutions contain 4 different combinations of active ingredients (7 different products with varying ingredient amounts). PHOXILLUM replacement solutions contain 2 different combinations of active ingredients (2 different products with varying ingredient amounts). PRISMASOL and PHOXILLUM are supplied in a two-compartment bag that must be mixed immediately prior to use
- Small compartment A (250 mL) containing an electrolyte solution, and
- Large compartment B (4750 mL) containing the buffer solution.
See Table 1for the concentrations of the active ingredients (after mixing) in these 9 different replacement solutions (total volume is 5 Liters).
Ca2+ mEq/L | HCO3- mEq/L | K+ mEq/L | Mg2+ mEq/L | Na+ mEq/L | HPO42- mmol/L | Cl- mEq/L | Lactate mEq/L | Dextrose mg/dL | Osmolarity mOsm/L | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+= calcium, HCO3-= bicarbonate, K+= potassium, Mg2+= magnesium, Na+= sodium, HPO42-= phosphate, Cl- = chloride; osmolarity is estimated | ||||||||||
PRISMASOL Replacement Solutions | ||||||||||
BGK0/2.5 | 2.5 | 32 | 0 | 1.5 | 140 | 0 | 109 | 3 | 100 | 292 |
BGK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 0 | 113 | 3 | 100 | 300 |
BGK2/3.5 | 3.5 | 32 | 2 | 1 | 140 | 0 | 111.5 | 3 | 100 | 296 |
BGK2/0 | 0 | 32 | 2 | 1 | 140 | 0 | 108 | 3 | 100 | 291 |
B22GK4/0 | 0 | 22 | 4 | 1.5 | 140 | 0 | 120.5 | 3 | 100 | 296 |
BGK4/0/1.2 | 0 | 32 | 4 | 1.2 | 140 | 0 | 110.2 | 3 | 100 | 295 |
BK0/0/1.2 | 0 | 32 | 0 | 1.2 | 140 | 0 | 106.2 | 3 | 0 | 282 |
PHOXILLUM Replacement Solutions | ||||||||||
BK4/2.5 | 2.5 | 32 | 4 | 1.5 | 140 | 1 | 114.5 | 0 | 0 | 294 |
B22K4/0 | 0 | 22 | 4 | 1.5 | 140 | 1 | 122 | 0 | 0 | 290 |
The mode of therapy, solute formulation, flow rates, and length of PRISMASOL and PHOXILLUM replacement therapy in CRRT should be established by a physician based on the patient’s clinical condition, blood concentration of phosphate and other electrolytes, acid-base and glucose balance. Administer either PRISMASOL or PHOXILLUM into the extracorporeal circuit:
- Before (pre-dilution) the hemofilter or hemodiafilter,
- After (post-dilution) the hemofilter or hemodiafilter, or
- Before and after the hemofilter or hemodiafilter.
PRISMASOL and PHOXILLUM are pharmacologically inactive solutions. While there are no adequate and well controlled studies in pregnant women, appropriate administration of PRISMASOL and PHOXILLUM solutions with monitoring of fluid, electrolyte, acid-base and glucose balance, is not expected to cause fetal harm. Animal reproduction studies have not been conducted with PRISMASOL and PHOXILLUM solutions.
The estimated background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
PHOXILLUM and PRISMASOL replacement solutions are contraindicated in patients with known hypersensitivities to these products.
- Monitor hemodynamic status and fluid inputs and outputs, potassium, phosphorus, other electrolytes and acid-base balance. Abnormalities may be corrected by the use of appropriate formulations and dosage of PRISMASOL and PHOXILLUM solutions ()
5.1 Electrolyte and Volume AbnormalitiesPHOXILLUM and PRISMASOL solutions can affect electrolytes and volume and may result in hyperkalemia or hyperphosphatemia. Monitor hemodynamic status and fluid inputs and outputs, potassium, phosphorous, calcium, other electrolytes and acid-base balance throughout the procedure. Abnormalities may be corrected by changing the formulation of replacement solution and/or dialysate, supplementation, or adjusting flow rates appropriately [
see Dosage and Administration (2)].PHOXILLUM replacement solutions contain hydrogen phosphate, a weak acid that may increase the risk of metabolic acidosis. - Treatment may affect glucose levels. Monitor blood glucose levels.
- Antidiabetic therapy adjustment or other corrective measures may be required during treatment ()
5.2 Blood Glucose AbnormalitiesThe use of PRISMASOL and PHOXILLUM replacement solutions can affect blood glucose levels resulting in hypo- or hyper-glycemia depending upon the dextrose content of the replacement solution. Monitor blood glucose levels regularly. Patients may require initiation of or modification of antidiabetic therapy or other corrective measures during treatment.