Promethazine Hydrochloride
Promethazine Hydrochloride Prescribing Information
- Promethazine hydrochloride tablets, USP are useful for:
- Perennial and seasonal allergic rhinitis
- Vasomotor rhinitis
- Allergic conjunctivitis due to inhalant allergens and foods
- Mild, uncomplicated allergic skin manifestations of urticarial and angioedema
- Amelioration of allergic reactions to blood or plasma
- Dermographism
- Anaphylactic reactions as adjunctive therapy to epinephrine and other standard measures after the acute manifestations have been controlled
- Preoperative, postoperative and obstetric sedation
- Prevention and control of nausea and vomiting associated with certain types of anesthesia and surgery.
- Therapy adjunctive to meperidine or other analgesics for control of postoperative pain.
- Sedation in both children and adults as well as relief of apprehension and production of light sleep from which the patient can be easily aroused.
- Active and prophylactic treatment of motion sickness
- Antiemetic therapy in postoperative patients
Promethazine hydrochloride tablets, USP Tablets may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a vehicle or operating machinery. The impairment may be amplified by concomitant use of other central-nervous-system depressants such as alcohol, sedatives/hypnotics (including barbiturates), narcotics, narcotic analgesics, general anesthetics, tricyclic antidepressants, and tranquilizers; therefore such agents should either be eliminated or given in reduced dosage in the presence of promethazine hydrochloride (see PRECAUTIONS, Information for Patientsand Drug Interactions).
Promethazine hydrochloride tablets, USP may lead to potentially fatal respiratory depression.
Use of a promethazine hydrochloride tablets, USP in patients with compromised respiratory function (e.g., COPD, sleep apnea) should be avoided.
Promethazine Hydrochloride Tablets, USP may lower seizure threshold. It should be used with caution in persons with seizure disorders or in persons who are using concomitant medications, such as narcotics or local anesthetics, which may also affect seizure threshold.
Promethazine hydrochloride tablets, USP, should be used with caution in patients with bone marrow depression. Leukopenia and agranulocytosis has been reported, usually when promethazine hydrochloride has been used in association with other known marrow-toxic agents.
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with promethazine hydrochloride alone or in combination with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of promethazine hydrochloride, antipsychotic drugs, if any, and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
Since reoccurrences of NMS have been reported with phenothiazines, the reintroduction of promethazine hydrochloride should be carefully considered.
Excessively large dosages of antihistamines, including promethazine hydrochloride tablets, USP in pediatric patients may cause sudden death (see OVERDOSAGE). Hallucinations and convulsions have occurred with therapeutic doses and overdoses of promethazine hydrochloride tablets, USP in pediatric patients. In pediatric patients who are acutely ill associated with dehydration, there is an increased susceptibility to dystonias with the use of Promethazine hydrochloride.
Administration of Promethazine hydrochloride has been associated with reported cholestatic jaundice.
Excessively large dosages of antihistamines, including promethazine hydrochloride tablets, USP in pediatric patients may cause sudden death (see OVERDOSAGE). Hallucinations and convulsions have occurred with therapeutic doses and overdoses of promethazine hydrochloride tablets, USP in pediatric patients. In pediatric patients who are acutely ill associated with dehydration, there is an increased susceptibility to dystonias with the use of Promethazine hydrochloride.
WARNINGS:
Excessively large dosages of antihistamines, including promethazine hydrochloride tablets, USP in pediatric patients may cause sudden death (see OVERDOSAGE). Hallucinations and convulsions have occurred with therapeutic doses and overdoses of promethazine hydrochloride tablets, USP in pediatric patients. In pediatric patients who are acutely ill associated with dehydration, there is an increased susceptibility to dystonias with the use of Promethazine hydrochloride.
The average effective dose ofpromethazine hydrochloride tablets, USP for the active therapy of nausea and vomiting in children or adults is 25mg. When oral medication cannot be tolerated, the dose should be given parenterally or by rectal suppository. 12.5 to 25mg doses may be repeated, as necessary, at four-to-six-hour intervals.
For nausea and vomiting in children, the usual dose is 0.5mg per pound of body weight, and the dose should be adjusted to the age and weight of the patient and the severity of the condition being treated.
For prophylaxis of nausea and vomiting, as during surgery and the postoperative period, the average dose is 25mg repeated at four-to-six-hour intervals, as necessary.
This product relieves apprehension and induces a quiet sleep from which the patient can be easily aroused. Administration of 12.5 to 25 mg promethazine hydrochloride by the oral route or by rectal suppository at bedtime will provide sedation in children. Adults usually require 25 to 50 mg for nighttime, pre-surgical, or obstetrical sedation.
Promethazine hydrochloride tablets, USP in 12.5 to 25 mg doses for children and 50 mg doses for adults the night before surgery relieves apprehension and produces a quiet sleep.
For preoperative medication, children require doses of 0.5mg per pound of body weight in combination with an appropriately reduced dose of narcotic or barbiturate and the appropriate dose of an atropine-like drug. Usual adult dosage is 50mg promethazine hydrochloride tablets, USP with an appropriately reduced dose of narcotic or barbiturate and the required amount of a belladonna alkaloid.
Postoperative Sedation and adjunctive use with analgesics may be obtained by the administration of 12.5 to 25mg in children and 25 to 50 mg doses in adults.
Promethazine hydrochloride tablets, USP are contraindicated for children under 2 years of age.
Promethazine Hydrochloride Tablets, USP are contraindicated for use in pediatric patients less than two years of age.
Promethazine hydrochloride tablets, USP are contraindicated in comatose state, and in individuals know to be hypersensitive or to have had an idiosyncratic reaction to promethazine or to other phenthiazines.
Antihistamines are contraindicated for use in the treatments of lower respiratory tract symptoms including asthma.
Promethazine hydrochloride tablets, USP may lead to potentially fatal respiratory depression.
Use of a promethazine hydrochloride tablets, USP in patients with compromised respiratory function (e.g., COPD, sleep apnea) should be avoided.
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with promethazine hydrochloride alone or in combination with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of promethazine hydrochloride, antipsychotic drugs, if any, and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
Since reoccurrences of NMS have been reported with phenothiazines, the reintroduction of promethazine hydrochloride should be carefully considered.
Hyperexcitability and abnormal movements have been reported in patients following a single administration of promethazine hydrochloride. Consideration should be given to the discontinuation of promethazine hydrochloride and to the use of other drugs if these reactions occur. Respiratory depression, nightmares, delirium, and agitated behavior have also been reported in some of these patients.
Each 25 mg promethazine hydrochloride tablet for oral administration contains 25 mg promethazine hydrochloride.
Each 50 mg promethazine hydrochloride tablet for oral administration contains 50 mg promethazine hydrochloride.
Each tablet for oral administration contains 25 mg or 50 mg promethazine hydrochloride, USP. The inactive ingredients include: lactose anhydrous, magnesium stearate, and microcrystalline cellulose. The 50 mg also contains D&C Red # 27 Lake.
Promethazine hydrochloride is a racemic compound; the empirical formula is C17H20N2S•HCl and its molecular weight is 320.88.
Promethazine hydrochloride, a phenothiazine derivative, is designated chemically as 10HPhenothiazine-10-ethanamine, N,N,a-trimethyl-, monohydrochloride, (±)- with the following structural formula:
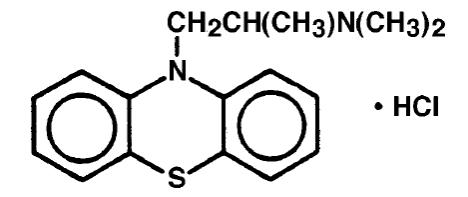
Promethazine hydrochloride occurs as a white to faint yellow, practically odorless, crystalline powder which slowly oxidizes and turns blue on prolonged exposure to air. It is freely soluble in water and soluble in alcohol.