Protopam Chloride
(Pralidoxime Chloride)Protopam Chloride Prescribing Information
PROTOPAM Chloride is indicated as an antidote:
1. In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have anticholinesterase activity and2. In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis.
The principal indications for the use of PROTOPAM Chloride are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness.
Treatment should include general supportive care, atropinization, and decontamination, in addition to the use of PROTOPAM Chloride. Treatment is most effective if initiated immediately after poisoning. Administration of PROTOPAM Chloride should be carried out slowly and, preferably, by infusion. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used. Generally, little is accomplished if PROTOPAM Chloride is given more than 36 hours after termination of exposure to the poison. When the poison has been ingested, it is particularly important to take into account the likelihood of continuing absorption from the lower bowel since this constitutes new exposure and fatal relapses have been reported after initial improvement. In such cases, additional doses of PROTOPAM Chloride may be needed every three to eight hours. In effect, the patient should be “titrated” with PROTOPAM Chloride as long as signs of poisoning recur. As in all cases of organophosphate poisoning, care should be taken to keep the patient under observation for at least 48 to 72 hours.
If dermal exposure has occurred, clothing should be removed and the hair and skin washed thoroughly with sodium bicarbonate or alcohol as soon as possible.
Supportive care, including airway management, respiratory and cardiovascular support, correction of metabolic abnormalities, and seizure control, may be necessary in cases of severe organophosphate poisoning.
Atropine should be given as soon as possible after hypoxemia is improved. Atropine should not be given in the presence of significant hypoxia due to the risk of atropine-induced ventricular fibrillation. In adults, atropine may be given intravenously in doses of 2 to 4 mg. This should be repeated at 5- to 10-minute intervals until full atropinization (secretions are inhibited) or signs of atropine toxicity appear (delirium, hyperthermia, muscle twitching).
Some degree of atropinization should be maintained for at least 48 hours, and until any depressed blood cholinesterase activity is reversed.
Use of morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning (see
When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
After the effects of atropine become apparent, PROTOPAM Chloride may be administered.
There are no known absolute contraindications for the use of PROTOPAM Chloride (see
When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
Treatment should include general supportive care, atropinization, and decontamination, in addition to the use of PROTOPAM Chloride. Treatment is most effective if initiated immediately after poisoning. Administration of PROTOPAM Chloride should be carried out slowly and, preferably, by infusion. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used. Generally, little is accomplished if PROTOPAM Chloride is given more than 36 hours after termination of exposure to the poison. When the poison has been ingested, it is particularly important to take into account the likelihood of continuing absorption from the lower bowel since this constitutes new exposure and fatal relapses have been reported after initial improvement. In such cases, additional doses of PROTOPAM Chloride may be needed every three to eight hours. In effect, the patient should be “titrated” with PROTOPAM Chloride as long as signs of poisoning recur. As in all cases of organophosphate poisoning, care should be taken to keep the patient under observation for at least 48 to 72 hours.
If dermal exposure has occurred, clothing should be removed and the hair and skin washed thoroughly with sodium bicarbonate or alcohol as soon as possible.
Supportive care, including airway management, respiratory and cardiovascular support, correction of metabolic abnormalities, and seizure control, may be necessary in cases of severe organophosphate poisoning.
Atropine should be given as soon as possible after hypoxemia is improved. Atropine should not be given in the presence of significant hypoxia due to the risk of atropine-induced ventricular fibrillation. In adults, atropine may be given intravenously in doses of 2 to 4 mg. This should be repeated at 5- to 10-minute intervals until full atropinization (secretions are inhibited) or signs of atropine toxicity appear (delirium, hyperthermia, muscle twitching).
Some degree of atropinization should be maintained for at least 48 hours, and until any depressed blood cholinesterase activity is reversed.
Use of morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning (see PRECAUTIONS, Drug Interactions). Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
After the effects of atropine become apparent, PROTOPAM Chloride may be administered.
PROTOPAM Chloride dosing is based, in part, on the severity of symptoms of nerve agent intoxication. These symptoms include the following:
MILD symptoms:
• Blurred vision and sore eyes• Teary eyes*• Runny nose*• Increased salivation such as sudden drooling*• Chest tightness or difficulty breathing• Tremors throughout the body or muscular twitching• Nausea and vomiting• Involuntary respiratory secretions
SEVERE symptoms:
• Strange or confused behavior• Severe difficulty breathing or respiratory secretions• Severe muscular twitching and general weakness**• Involuntary urination and defecation*• Convulsions• Unconsciousness
* These symptoms are sometimes observed in healthy infants and young children. In this age group, these symptoms are less reliable than other symptoms listed. Symptoms must be considered collectively when nerve agent or pesticide exposure is known or suspected.
** Infants may become drowsy or unconscious, with muscle floppiness rather than muscle twitching, soon after exposure to nerve agents or pesticides.
Refer to the Preparation for Administrationsection for instructions on reconstitution and dilution of PROTOPAM Chloride that result in a 10-20 mg/mL solution for intravenous infusion.
Inject an initial dose of 1000 to 2000 mg of PROTOPAM Chloride, preferably as an infusion in 100 mL of normal saline, over a 15- to 30-minute period. If this is not practical or if pulmonary edema is present, the dose should be given slowly (over not less than five minutes) by intravenous injection, as a 50 mg/mL solution in water (e.g., 1000 mg in 20 mL). A second dose of 1000 to 2000 mg may be indicated after about one hour if muscle weakness has not been relieved. Additional doses may be given every 10-12 hours if muscle weakness persists.
Intravenous administration of PROTOPAM Chloride should be carried out slowly and, preferably, by continuous or intermittent infusion, since temporary worsening of cholinergic manifestations (i.e. tachycardia, cardiac arrest, laryngospasm, and muscle rigidity or paralysis) may occur if PROTOPAM Chloride is infused too rapidly. The intermittent infusion rate should not exceed 200 mg/minute. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used.
Evidence suggests that a loading dose followed by continuous intravenous infusion of PROTOPAM Chloride may maintain therapeutic levels longer than traditional short intermittent infusions therapy (see Pharmacokinetics).
Refer to the Preparation for Administrationsection for instructions on reconstitution of PROTOPAM Chloride that result in an approximate 300 mg/mL solution for intramuscular administration.
Intramuscular dosing in adults should be based on the severity of clinical symptoms.
MILD SYMPTOMS
• For treatment of mild symptoms, administer a 600 mg (2 mL) intramuscular dose of PROTOPAM Chloride. Wait 15 minutes for PROTOPAM Chloride to take effect.• If, after 15 minutes, mild symptoms persist, then administer a second 600 mg (2 mL) intramuscular dose of PROTOPAM Chloride.• If, after an additional 15 minutes, mild symptoms continue to persist, a third 600 mg (2 mL) dose of PROTOPAM Chloride may be administered for a total cumulative dose of 1800 mg.• If at any time after the first dose, the patient develops severe symptoms, administer two additional 600 mg intramuscular doses in rapid succession for a total cumulative dose of 1800 mg of PROTOPAM Chloride.
SEVERE SYMPTOMS
• For treatment of severe symptoms, administer three 600 mg intramuscular doses (3 doses of 2 mL each) in rapid succession for a total dose of 1800 mg of PROTOPAM Chloride.
PERSISTENT SYMPTOMS
• If symptoms persist after administering the complete 1800 mg regimen (3 injections of 600 mg each), the series may be repeated beginning approximately 1 hour after administration of the last injection.
Refer to the Preparation for Administrationsection for instructions on reconstitution and dilution of PROTOPAM Chloride that result in 10-20 mg/mL solution for intravenous infusion.
PROTOPAM Chloride can be given as intermittent intravenous infusions or as a loading dose followed by continuous intravenous infusion, depending upon the patient’s clinical condition. The specific dose given should depend upon the severity of the symptoms.
Administer a loading dose of 20-50 mg/kg (not to exceed 2000 mg/dose) over 15-30 minutes followed by a continuous infusion of 10-20 mg/kg/hour.
Administer an initial intermittent infusion of 20-50 mg/kg (not to exceed 2000 mg/dose) over 15-30 minutes. A second dose of 20-50 mg/kg may be indicated after about one hour if muscle weakness has not been relieved. Repeat dosing is permissible every 10-12 hours as needed.
If it is not practical to administer intermittent or continuous intravenous infusions, or if pulmonary edema is present, the 20-50 mg/kg dose should be given slowly (over not less than five minutes) by intravenous injection as a 50 mg/mL solution in water (see Preparation for Administrationsection). Additional doses may be given every 10-12 hours if muscle weakness persists.
Refer to the Preparation for Administrationsection for instructions on reconstitution of PROTOPAM Chloride that result in an approximate 300 mg/mL solution for intramuscular administration.
Intramuscular injections in children should be administered in the anterolateral aspect of the thigh to avoid the nerve, artery and vein, as well as the femur.
Pharmacokinetic modeling using published data from the scientific literature was conducted to derive intramuscular dosing recommendations in the pediatric population. The specific intramuscular dose of PROTOPAM Chloride should depend upon the severity of the symptoms.
MILD SYMPTOMS
• For the treatment of mild symptoms, administer a weight-appropriate intramuscular dose (see Table 1 below) of PROTOPAM Chloride. Wait 15 minutes for PROTOPAM Chloride to take effect.• If, after 15 minutes, mild symptoms persist, then administer a second weight-appropriate intramuscular dose of PROTOPAM Chloride.• If after an additional 15 minutes, mild symptoms continue to persist, a third weight-appropriate intramuscular dose of PROTOPAM Chloride may be administered.• The three PROTOPAM Chloride injections together are considered a single course of treatment, and the total amount of PROTOPAM Chloride administered per course of treatment (i.e., 3 weight-appropriate injections) should not exceed the total amounts listed in Table 1 below.• If at any time after the first dose, the patient develops severe symptoms, administer two additional weight-appropriate intramuscular doses of PROTOPAM Chloride in rapid succession.
SEVERE SYMPTOMS
• For treatment of severe symptoms, administer the weight-appropriate intramuscular dose (see Table 1 below) of PROTOPAM Chloride Injection as three injections, in rapid succession, into the patient’s anterolateral thigh (see Table 1 below).
PERSISTENT SYMPTOMS
• If symptoms persist after administering a complete course (3 injections of the weight-appropriate dose each), the series may be repeated beginning approximately 1 hour after administration of the last injection.
Weight in kg | Dose Per Injection During the treatment of mild symptoms, if at any time after the first dose, the patient develops severe symptoms, administer two additional weight-appropriate intramuscular doses of PROTOPAM Chloride in rapid succession. | Total Dose per Three-Injection Course Additional courses of PROTOPAM Chloride may be administered beginning one hour after the last injection. A single course consists of three separate, weight-appropriate injections, administered either with 15 minute inter-injection observation periods for patients with mild symptoms, or all in rapid succession for patients with severe symptoms. |
< 40kg | 15 mg/kg | 45 mg/kg |
≥ 40 kgWeight of 40 kg corresponds to approximately the 50th percentile for a 12 year old child per the weight-for-age percentile growth charts published by the Centers for Disease Control and Prevention in 2000. | Use Adult Dosing RecommendationsAdult Dose Per Injection is 600 mg; Total Adult Dose per Three-Injection Course is 1800 mg. | Use Adult Dosing Recommendations |
As an antagonist to such anticholinesterases as neostigmine, pyridostigmine, and ambenonium, which are used in the treatment of myasthenia gravis, PROTOPAM Chloride may be given in a dosage of 1000 to 2000 mg intravenously followed by increments of 250 mg every five minutes.
PROTOPAM Chloride is supplied as 1000 mg single-dose vials for injection.
For INTRAVENOUS infusion: Reconstitute a single PROTOPAM Chloride 1000 mg vial by adding 20 mL of Sterile Water for Injection, USP, which results in a 50 mg/mL concentration.
The solution should further be diluted with Normal Saline for Injection, USP to achieve a concentration of
For fluid restricted patients or for rapid administration (over at least 5 min), a maximum concentration of 50 mg/mL may be used.
For INTRAMUSCULAR injection: Reconstitute a single PROTOPAM Chloride 1000 mg vial by adding 3.3 mL of Sterile Water for Injection, USP for an approximate concentration of
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard unused solution after a dose has been withdrawn.
Forty to 60 minutes after intramuscular injection, mild to moderate pain may be experienced at the site of injection.
Pralidoxime chloride may cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure, hyperventilation, and muscular weakness when given parenterally to normal volunteers who have not been exposed to anticholinesterase poisons. In patients, it is very difficult to differentiate the toxic effects produced by atropine or the organophosphate compounds from those of the drug.
Elevations in SGOT and/or SGPT enzyme levels were observed in 1 of 6 normal volunteers given 1200 mg of pralidoxime chloride intramuscularly, and in 4 of 6 volunteers given 1800 mg intramuscularly. Levels returned to normal in about 2 weeks. Transient elevations in creatine phosphokinase were observed in all normal volunteers given the drug.
When atropine and pralidoxime chloride are used together, the signs of atropinization may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed. Excitement and manic behavior immediately following recovery of consciousness have been reported in several cases. However, similar behavior has occurred in cases of organophosphate poisoning that were not treated with pralidoxime chloride.
When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
Chemical name: 2-formyl-1-methylpyridinium chloride oxime. Available in the United States as PROTOPAM Chloride for Injection (PROTOPAM Chloride), pralidoxime chloride is frequently referred to as 2-PAM Chloride.
Structural formula:
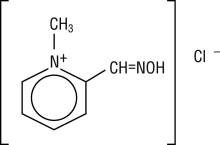
Pralidoxime chloride occurs as an odorless, white, nonhygroscopic, crystalline powder which is soluble in water. Stable in air, it melts between 215º and 225º C, with decomposition.
The specific activity of the drug resides in the 2-formyl-1-methylpyridinium ion and is independent of the particular salt employed. The chloride is preferred because of physiologic compatibility, excellent water solubility at all temperatures, and high potency per gram, due to its low molecular weight.
Pralidoxime chloride is a cholinesterase reactivator.
PROTOPAM Chloride for intravenous injection or infusion is prepared by cryo-desiccation. Each vial contains 1000 mg of sterile pralidoxime chloride, and sodium hydroxide to adjust pH, to be reconstituted with 20 mL of Sterile Water for Injection, USP. The pH of the reconstituted solution is 3.5 to 4.5. Intramuscular or subcutaneous injection may be used when intravenous injection is not feasible.