Pyridostigmine Bromide
Pyridostigmine Bromide Prescribing Information
Pyridostigmine bromide is available in one dosage form.
Dosage
Extended-release Tablets
Pyridostigmine bromide is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
The side effects of Pyridostigmine bromide are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharmaceuticals, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch.
Pyridostigmine bromide is an orally active cholinesterase inhibitor. Chemically, Pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:
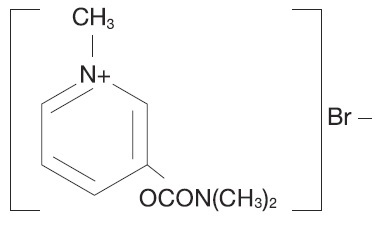
Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin®), but differs from it in certain clinically significant respects, for example, Pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
Pyridostigmine bromide is useful in the treatment of myasthenia gravis.
Extended-release Tablets are available as light straw colored capsule-shaped tablets containing 180 mg Pyridostigmine bromide in bottles of 30 (NDC 64980-220-03). Each tablet is engraved "NM 180" on one side and is single-scored on the other.
Store Pyridostigmine Bromide Extended-Release Tablets, 180 mg at 25°C (77°F), excursions permitted to 15°C-30°C (59°F-86°F). Keep Pyridostigmine Bromide Extended-Release Tablets, 180 mg in a dry place with the silica gel enclosed.