Pyridoxine Hydrochloride
Pyridoxine Hydrochloride Prescribing Information
Pyridoxine Hydrochloride Injection is effective for the treatment of pyridoxine deficiency as seen in the following:
Inadequate dietary intake.
Drug-induced deficiency, as from isoniazid (INH) or oral contraceptives.
Inborn errors of metabolism, e.g., vitamin B
6 dependent convulsions or vitamin B
6 responsive anemia.
The parenteral route is indicated when oral administration is not feasible as in anorexia, nausea and vomiting, and preoperative and postoperative conditions. It is also indicated when gastrointestinal absorption is impaired.
Pyridoxine Hydrochloride Injection may be administered intramuscularly or intravenously. In cases of dietary deficiency, the dosage is 10 to 20 mg daily for 3 weeks. Follow-up treatment is recommended daily for several weeks with an oral therapeutic multivitamin preparation containing 2 to 5 mg pyridoxine. Poor dietary habits should be corrected, and an adequate, well balanced diet should be prescribed.
The vitamin B
6 dependency syndrome may require a therapeutic dosage of as much as 600 mg a day and a daily intake of 30 mg for life.
In deficiencies due to INH, the dosage is 100 mg daily for 3 weeks followed by a 30 mg maintenance dose daily.
In poisoning caused by ingestion of more than 10 g of INH, an equal amount of pyridoxine should be given — 4 g intravenously followed by 1 g intramuscularly every 30 minutes.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
A history of sensitivity to pyridoxine or to any of the ingredients in Pyridoxine Hydrochloride Injection, USP is a contraindication.
Paresthesia, somnolence, and low serum folic acid levels have been reported.
Pyridoxine supplements should not be given to patients receiving levodopa, because the action of the latter drug is antagonized by pyridoxine. However, this vitamin may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
Pyridoxine Hydrochloride Injection, USP is a sterile solution of pyridoxine hydrochloride in Water for Injection. Each mL contains 100 mg pyridoxine hydrochloride and 0.5% chlorobutanol anhydrous (chloral deriv.). pH adjusted with sodium hydroxide if necessary (2.0 to 3.8).
Pyridoxine hydrochloride is a colorless or white crystal or a white crystalline powder. One gram dissolves in 5 mL of water. It is stable in air and is slowly affected by sunlight.
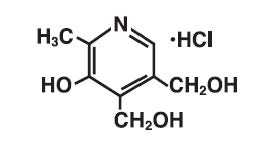
The chemical name is 2-methyl-3-hydroxy-4,5-bis (hydroxymethyl) pyridine hydrochloride.
The structural formula is: