R-gene
(Arginine Hydrochloride)R-Gene Prescribing Information
R-Gene® 10 is indicated as an intravenous stimulant to the pituitary for the release of human growth hormone in patients where the measurement of pituitary reserve for HGH can be of diagnostic usefulness. It can be used as a diagnostic aid in such conditions as panhypopituitarism, pituitary dwarfism, chromophobe adenoma, postsurgical craniopharyngioma, hypophysectomy, pituitary trauma, acromegaly, gigantism and problems of growth and stature.
If the insulin hypoglycemia test has indicated a deficiency of pituitary reserve for HGH, a test with R-Gene® 10 is advisable to confirm the negative response. This can be done after a waiting period of one day. As patients may not respond to R-Gene® 10 (Arginine Hydrochloride Injection, USP) during the first test, the unresponsive patient should be tested again to confirm the negative result. A second test can be performed after a waiting period of one day. Some patients who respond to R-Gene® 10 do not respond to insulin and vice versa. The rate of false positive responses for R-Gene® 10 is approximately 32%, and the rate of false negatives is approximately 27%.
The recommended adult dose is 30 g arginine hydrochloride (300 mL of R-Gene® 10) administered by intravenous infusion over 30 minutes. The total dose should not exceed 30 g arginine hydrochloride. See
R-Gene®10 is provided as a ready-to-use solution for patients weighing 60 kg (132 lbs) or more and should not be further diluted. For pediatric patients weighing 59 kg (130 lbs) or less a dose must be placed in a separate container. Follow the preparation instructions below.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Withdraw a weight-based dose from an intact sealed bottle of R-Gene®10. The entire 300 mL bottle of R-Gene®10 for infusion is not intended for use in patients weighing 59 kg or less. The dose must be placed in a separate container, such as an evacuated sterile glass container designed for intravenous administration, using aseptic technique.
Additionally, R-Gene®10 is stable in polypropylene syringes and plastic containers made of either polyvinyl chloride (PVC) or ethylene vinyl acetate (EVA).
The post-penetration storage period is not more than 4 hours including infusion time at room temperature or 24 hours at refrigerated temperature (2-8°C).
The healthcare professional administering the dose should verify the accuracy of the dose prior to administration.
Use only if the solution is clear. Discard any unused drug product.
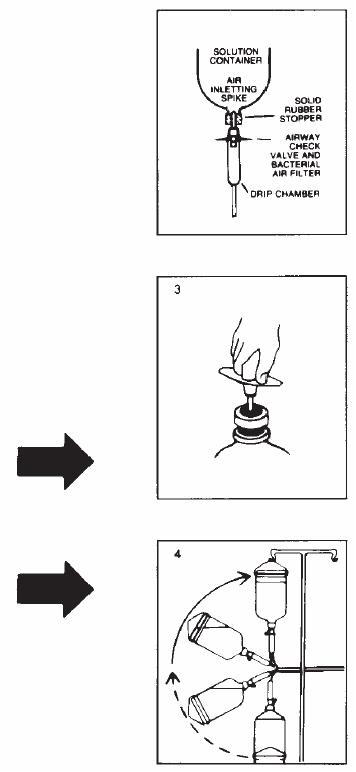
Follow these directions using aseptic technique. As R-Gene®10 for intravenous use is provided in glass containers, a standard air-inletting, air-filtering intravenous infusion set with a bacterial air filter is required.
1.000000000000000e+00 Use only if solution is clear and seal is intact.Carefully examine bottle for evidence of damage, e.g., small cracks, dents in seal, or areas of dried powder on exterior.Do not administer contents if such damage is found.2.000000000000000e+00 Remove plastic flip off lid from bottle to expose rubber stopper, taking care that you do not contaminate the target site of the stopper with fingers, hair, clothing, etc.Immediatelyperform step #3.3.000000000000000e+00 With shut-off clamp closed, remove sterility protector from spike of administration set and immediately insert set with a quick thrust into center of stopper with bottle upright on table. (Push straight in — don't twist — twisting may cause stopper coring.)4.000000000000000e+00 Promptly invert bottle to automatically establish fluid level in drip chamber and to check for vacuum by observing rising filtered air bubbles.Discard bottle if there is no vacuum or if the solution is not clear.5.000000000000000e+00 Clear tubing of air. Proceed with infusion.

The administration of R-Gene® 10 is contraindicated in persons having known hypersensitivity to any ingredient in this product.
Adverse reactions associated with 1670 infusions in premarketing studies were as follows:
Non-specific side effects consisting of nausea, vomiting, headache, flushing, numbness and local venous irritation were reported in approximately 3% of the patients.
One patient had an allergic reaction which was manifested as a confluent macular rash with reddening and swelling of the hands and face. The rash subsided rapidly after the infusion was terminated and 50 mg of diphenhydramine were administered. One patient had an apparent decrease in platelet count from 150,000 to 60,000. One patient with a history of acrocyanosis had an exacerbation of this condition following infusion of R-Gene® 10.
Each 100 mL of R-Gene® 10 (Arginine Hydrochloride Injection, USP) for intravenous use contains 10 g of L-Arginine Hydrochloride, USP in Water for Injection, USP (equivalent to a 10% solution) (0.1 g/mL). L-arginine is a naturally occurring amino acid.
R-Gene® 10 is hypertonic (950 mOsmol/liter) and contains 47.5 mEq of chloride ion per 100 mL of solution. The pH is adjusted to 5.6 (5.0–6.5) with arginine base or hydrochloric acid.
Intravenous infusion of R-Gene® 10 often induces a pronounced rise in the plasma level of human growth hormone (HGH) in subjects with intact pituitary function. This rise is usually diminished or absent in patients with impairment of this function.
Patient | Control Range | Range of Peak Response to Arginine |
|---|---|---|
Normal | 0–6 | 10–30 |
Pituitary | ||
deficient | 0–4 | 0–10 |
These ranges are based on the mean values of plasma HGH levels calculated from the data of several clinical investigators and reflect their experiences with various methods of radioimmunoassay. Upon gaining experience with this diagnostic test, each clinician will establish his/her own ranges for control and peak levels of HGH.
L-arginine is a normal metabolite in animals and man and has a low order of toxicity.