Ranitidine - Ranitidine tablet
(Ranitidine)Ranitidine - Ranitidine tablet Prescribing Information
Ranitidine Tablets, USP are indicated in:
- Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
- The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
- Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
- Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
- Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with Ranitidine Tablets, USP 150 mg b.i.d.
- Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with Ranitidine Tablets, USP 150 mg q.i.d.
- Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
Ranitidine Tablets, USP are a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP do not lower serum Ca++in hypercalcemic states. Ranitidine Tablets, USP are not an anticholinergic agent.
Patients With Impaired Renal Function
Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing | ||||
| Population (age) | n | Dosage Form (dose) | Cmax (ng/mL | Tmax (hours) |
| Gastric or duodenal ulcer (3.5 to 16 years) | 12 | Tablets (1 to 2 mg/kg) | 54 to 492 | 2 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Single dose) | 10 | Syrup (2 mg/kg) | 244 | 1.61 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Multiple dose) | 10 | Syrup (2 mg/kg) | 320 | 1.66 |
Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see
Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion | |||||
| Time After Dose, hours | % Inhibition of Gastric Acid Output by Dose, mg | ||||
| 75 to 80 | 100 | 150 | 200 | ||
| Basal | Up to 4 | 99 | 95 | ||
| Nocturnal | Up to 13 | 95 | 96 | 92 | |
| Betazole | Up to 3 | 97 | 99 | ||
| Pentagastrin | Up to 5 | 58 | 72 | 72 | 80 |
| Meal | Up to 3 | 73 | 79 | 95 | |
It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are more difficult to suppress.
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen, or estrogen levels.
- No antiandrogenic action.
- No effect on count, motility, or morphology of sperm.
Table 3. Duodenal Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP * | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 195 | 69/182 (38%)† | 188 | 31-164 (19%) |
| Week 2 | ||||
| Week 4 | 137/187 (73%)† | 76/168 (45%) | ||
*All patients were permitted antacids as needed for relief of pain.
†P<0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
Table 4. Mean Daily Doses of Antacid | ||
| Ulcer Healed | Ulcer Not Healed | |
| Ranitidine | 0.06 | 0.71 |
| Placebo | 0.71 | 1.43 |
Foreign studies have shown that patients heal equally well with 150 mg twice daily and 300 mg at bedtime (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg twice daily as compared to 300 mg at bedtime (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Table 5. Duodenal Ulcer Prevalence | |||||
| Double-Blind, Multicenter, Placebo-Controlled Trials | |||||
| Multicenter Trial | Drug | Duodenal Ulcer Prevalence | No. of Patients | ||
| 0 to 4 Months | 0 to 8 Months | 0 to 12 Months | |||
| USA | RAN | 20%* | 24%* | 35%* | 138 |
| PLC | 44% | 54% | 59% | 139 | |
| Foreign | RAN | 12%* | 21%* | 28%* | 174 |
| PLC | 56% | 64% | 68% | 165 | |
% = Life table estimate.
* =
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H2-antagonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Table 6. Gastric Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP* | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 92 | 16/83 (19%)† | 94 | 10/83 (12%) |
| Week 2 | ||||
| Week 6 | 50/73 (68%)† | 35/69 (51%) | ||
*All patients were permitted antacids as needed for relief of pain.
†
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
The US trial indicated that Ranitidine Tablets, USP 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In two additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg twice daily was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
The erosive esophagitis healing rates were as follows:
Table 7. Erosive Esophagitis Patient Healing Rates | ||
| Healed / Evaluable | ||
| Placebo* n=229 | Ranitidine Tablets, USP 150 mg 4 times daily* n=215 | |
| Week 4 | 43/198 (22%) | 96/206 (47%)† |
| Week 8 | 63/176 (36%) | 142/200 (71%)† |
| Week 12 | 92/159 (58%) | 162/192 (84%)† |
*All patients were permitted antacids as needed for relief of pain.
†
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg 4 times daily.
The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
The following 3 subsections provide dosing information for each of the pediatric indications.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Ranitidine Tablets, USP are a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP do not lower serum Ca++in hypercalcemic states. Ranitidine Tablets, USP are not an anticholinergic agent.
Patients With Impaired Renal Function
Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing | ||||
| Population (age) | n | Dosage Form (dose) | Cmax (ng/mL | Tmax (hours) |
| Gastric or duodenal ulcer (3.5 to 16 years) | 12 | Tablets (1 to 2 mg/kg) | 54 to 492 | 2 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Single dose) | 10 | Syrup (2 mg/kg) | 244 | 1.61 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Multiple dose) | 10 | Syrup (2 mg/kg) | 320 | 1.66 |
Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see
Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion | |||||
| Time After Dose, hours | % Inhibition of Gastric Acid Output by Dose, mg | ||||
| 75 to 80 | 100 | 150 | 200 | ||
| Basal | Up to 4 | 99 | 95 | ||
| Nocturnal | Up to 13 | 95 | 96 | 92 | |
| Betazole | Up to 3 | 97 | 99 | ||
| Pentagastrin | Up to 5 | 58 | 72 | 72 | 80 |
| Meal | Up to 3 | 73 | 79 | 95 | |
It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are more difficult to suppress.
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen, or estrogen levels.
- No antiandrogenic action.
- No effect on count, motility, or morphology of sperm.
Table 3. Duodenal Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP * | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 195 | 69/182 (38%)† | 188 | 31-164 (19%) |
| Week 2 | ||||
| Week 4 | 137/187 (73%)† | 76/168 (45%) | ||
*All patients were permitted antacids as needed for relief of pain.
†P<0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
Table 4. Mean Daily Doses of Antacid | ||
| Ulcer Healed | Ulcer Not Healed | |
| Ranitidine | 0.06 | 0.71 |
| Placebo | 0.71 | 1.43 |
Foreign studies have shown that patients heal equally well with 150 mg twice daily and 300 mg at bedtime (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg twice daily as compared to 300 mg at bedtime (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Table 5. Duodenal Ulcer Prevalence | |||||
| Double-Blind, Multicenter, Placebo-Controlled Trials | |||||
| Multicenter Trial | Drug | Duodenal Ulcer Prevalence | No. of Patients | ||
| 0 to 4 Months | 0 to 8 Months | 0 to 12 Months | |||
| USA | RAN | 20%* | 24%* | 35%* | 138 |
| PLC | 44% | 54% | 59% | 139 | |
| Foreign | RAN | 12%* | 21%* | 28%* | 174 |
| PLC | 56% | 64% | 68% | 165 | |
% = Life table estimate.
* =
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H2-antagonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Table 6. Gastric Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP* | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 92 | 16/83 (19%)† | 94 | 10/83 (12%) |
| Week 2 | ||||
| Week 6 | 50/73 (68%)† | 35/69 (51%) | ||
*All patients were permitted antacids as needed for relief of pain.
†
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
The US trial indicated that Ranitidine Tablets, USP 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In two additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg twice daily was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
The erosive esophagitis healing rates were as follows:
Table 7. Erosive Esophagitis Patient Healing Rates | ||
| Healed / Evaluable | ||
| Placebo* n=229 | Ranitidine Tablets, USP 150 mg 4 times daily* n=215 | |
| Week 4 | 43/198 (22%) | 96/206 (47%)† |
| Week 8 | 63/176 (36%) | 142/200 (71%)† |
| Week 12 | 92/159 (58%) | 162/192 (84%)† |
*All patients were permitted antacids as needed for relief of pain.
†
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg 4 times daily.
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (seeDOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Ranitidine was not mutagenic in standard bacterial tests
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Renal Function
Ranitidine Tablets, USP are contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients (see
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (seeDOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Ranitidine was not mutagenic in standard bacterial tests
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Renal Function
The following have been reported as events in clinical trials or in the routine management of patients treated with Ranitidine Tablets, USP. The relationship to therapy with Ranitidine Tablets, USP has been unclear in many cases. Headache, sometimes severe, seems to be related to administration of Ranitidine Tablets, USP.
Rare cases of breast symptoms and conditions, including galactorrhea and gynecomastia, have been reported in both males and females.
The active ingredient in Ranitidine Tablets, USP 150 mg and Ranitidine Tablets, USP 300 mg is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structure:
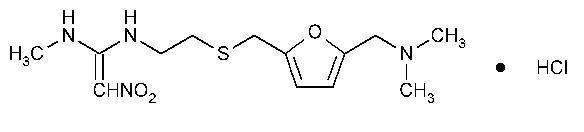
The empirical formula is C13H22N4O3S·HCl, representing a molecular weight of 350.87. Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfurlike odor.
Each Ranitidine Tablets, USP 150 mg for oral administration contains 167.4 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide, triethyl citrate and FD&C Yellow #6.
Each Ranitidine Tablets, USP 300 mg for oral administration contains 334.8 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide, triethyl citrate and D&C Yellow #10.
Ranitidine Tablets, USP are a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP do not lower serum Ca++ in hypercalcemic states. Ranitidine Tablets, USP are not an anticholinergic agent.
The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
The following 3 subsections provide dosing information for each of the pediatric indications.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (seeDOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Ranitidine was not mutagenic in standard bacterial tests
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Renal Function
The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
The following 3 subsections provide dosing information for each of the pediatric indications.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see
The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
The following 3 subsections provide dosing information for each of the pediatric indications.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see
| Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing | ||||
| Population (age) | n | Dosage Form (dose) | Cmax (ng/mL | Tmax (hours) |
| Gastric or duodenal ulcer (3.5 to 16 years) | 12 | Tablets (1 to 2 mg/kg) | 54 to 492 | 2 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Single dose) | 10 | Syrup (2 mg/kg) | 244 | 1.61 |
| Otherwise healthy requiring Ranitidine (0.7 to 14 years, Multiple dose) | 10 | Syrup (2 mg/kg) | 320 | 1.66 |
Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (seeDOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Ranitidine was not mutagenic in standard bacterial tests
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Renal Function
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (seeDOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Ranitidine was not mutagenic in standard bacterial tests
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see
Renal Function
The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
The following 3 subsections provide dosing information for each of the pediatric indications.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see
| Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion | |||||
| Time After Dose, hours | % Inhibition of Gastric Acid Output by Dose, mg | ||||
| 75 to 80 | 100 | 150 | 200 | ||
| Basal | Up to 4 | 99 | 95 | ||
| Nocturnal | Up to 13 | 95 | 96 | 92 | |
| Betazole | Up to 3 | 97 | 99 | ||
| Pentagastrin | Up to 5 | 58 | 72 | 72 | 80 |
| Meal | Up to 3 | 73 | 79 | 95 | |
It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are more difficult to suppress.
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen, or estrogen levels.
- No antiandrogenic action.
- No effect on count, motility, or morphology of sperm.
| Table 3. Duodenal Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP * | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 195 | 69/182 (38%) † | 188 | 31-164 (19%) |
| Week 2 | ||||
| Week 4 | 137/187 (73%) † | 76/168 (45%) | ||
*All patients were permitted antacids as needed for relief of pain.
†P<0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
| Table 4. Mean Daily Doses of Antacid | ||
| Ulcer Healed | Ulcer Not Healed | |
| Ranitidine | 0.06 | 0.71 |
| Placebo | 0.71 | 1.43 |
Foreign studies have shown that patients heal equally well with 150 mg twice daily and 300 mg at bedtime (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg twice daily as compared to 300 mg at bedtime (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
| Table 5. Duodenal Ulcer Prevalence | |||||
| Double-Blind, Multicenter, Placebo-Controlled Trials | |||||
| Multicenter Trial | Drug | Duodenal Ulcer Prevalence | No. of Patients | ||
| 0 to 4 Months | 0 to 8 Months | 0 to 12 Months | |||
| USA | RAN | 20%* | 24%* | 35%* | 138 |
| PLC | 44% | 54% | 59% | 139 | |
| Foreign | RAN | 12%* | 21%* | 28%* | 174 |
| PLC | 56% | 64% | 68% | 165 | |
% = Life table estimate.
* =
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H2-antagonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
| Table 6. Gastric Ulcer Patient Healing Rates | ||||
| Ranitidine Tablets, USP* | Placebo* | |||
| Number Entered | Healed / Evaluable | Number Entered | Healed / Evaluable | |
| Outpatients | 92 | 16/83 (19%) † | 94 | 10/83 (12%) |
| Week 2 | ||||
| Week 6 | 50/73 (68%) † | 35/69 (51%) | ||
*All patients were permitted antacids as needed for relief of pain.
†
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
The US trial indicated that Ranitidine Tablets, USP 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In two additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg twice daily was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
The erosive esophagitis healing rates were as follows:
| Table 7. Erosive Esophagitis Patient Healing Rates | ||
| Healed / Evaluable | ||
| Placebo* n=229 | Ranitidine Tablets, USP 150 mg 4 times daily* n=215 | |
| Week 4 | 43/198 (22%) | 96/206 (47%) † |
| Week 8 | 63/176 (36%) | 142/200 (71%) † |
| Week 12 | 92/159 (58%) | 162/192 (84%) † |
*All patients were permitted antacids as needed for relief of pain.
†
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg 4 times daily.