Relpax
(Eletriptan Hydrobromide)Relpax Prescribing Information
RELPAX is indicated for the acute treatment of migraine with or without aura in adults.
• Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine attack treated with RELPAX, reconsider the diagnosis of migraine before RELPAX is administered to treat any subsequent attacks.• RELPAX is not intended for the prevention of migraine attacks.• Safety and effectiveness of RELPAX have not been established for cluster headache.
The maximum recommended single dose is 40 mg.
In controlled clinical trials, single doses of 20 mg and 40 mg were effective for the acute treatment of migraine in adults. A greater proportion of patients had a response following a 40 mg dose than following a 20 mg dose
The efficacy of RELPAX in the acute treatment of migraines was evaluated in eight randomized, double-blind placebo-controlled studies. All eight studies used 40 mg. Seven studies evaluated an 80 mg dose and two studies included a 20 mg dose.
In all eight studies, randomized patients treated their headaches as outpatients. Seven studies enrolled adults and one study enrolled adolescents (ages 11 to 17). Patients treated in the seven adult studies were predominantly female (85%) and Caucasian (94%) with a mean age of 40 years (range 18 to 78). In all studies, patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed up to 2 hours after dosing. Associated symptoms such as nausea, vomiting, photophobia and phonophobia were also assessed.
Maintenance of response was assessed for up to 24 hours post dose. In the adult studies, a second dose of RELPAX or other medication was allowed 2 to 24 hours after the initial treatment for both persistent and recurrent headaches. The incidence and time to use of these additional treatments were also recorded.
In the seven adult studies, the percentage of patients achieving headache response 2 hours after treatment was significantly greater among patients receiving RELPAX at all doses compared to those who received placebo. The 2-hour response rates from these controlled clinical studies are summarized in Table 2.
Placebo | RELPAX 20 mg | RELPAX 40 mg | RELPAX 80 mg | |
|---|---|---|---|---|
| NA - Not Applicable | ||||
Study 1 | 23.8% | 54.3%p value < 0.05 vs placebo | 65.0% (n=117) | 77.1% (n=118) |
Study 2 | 19.0% | NA | 61.6% (n=430) | 64.6% (n=446) |
Study 3 | 21.7% | 47.3% (n=273) | 61.9% (n=281) | 58.6% (n=290) |
Study 4 | 39.5% | NA | 62.3% (n=175) | 70.0% (n=170) |
Study 5 | 20.6% | NA | 53.9% (n=206) | 67.9% (n=209) |
Study 6 | 31.3% | NA | 63.9% (n=169) | 66.9% (n=160) |
Study 7 | 29.5% | NA | 57.5% (n=492) | NA |
Comparisons of the performance of different drugs based upon results obtained in different clinical trials are never reliable. Because studies are generally conducted at different times, with different samples of patients, by different investigators, employing different criteria and/or different interpretations of the same criteria, under different conditions (dose, dosing regimen, etc.), quantitative estimates of treatment response and the timing of response may be expected to vary considerably from study to study.
The estimated probability of achieving an initial headache response within 2 hours following treatment is depicted in Figure 1.
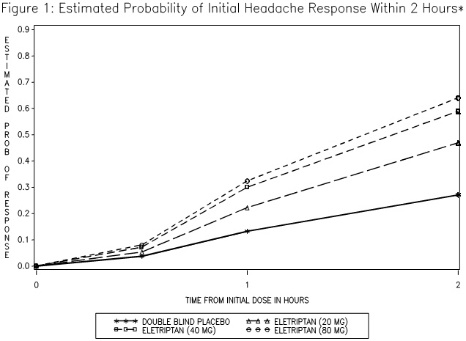
*Figure 1 shows the Kaplan-Meier plot of probability over time of obtaining headache response (no or mild pain) following treatment with eletriptan. The plot is based on 7 placebo-controlled, outpatient trials in adults providing evidence of efficacy (Studies 1 through 7). Patients not achieving headache response or taking additional treatment prior to 2 hours were censored at 2 hours.
For patients with migraine-associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of RELPAX as compared to placebo.
Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of taking a second dose or other medications for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
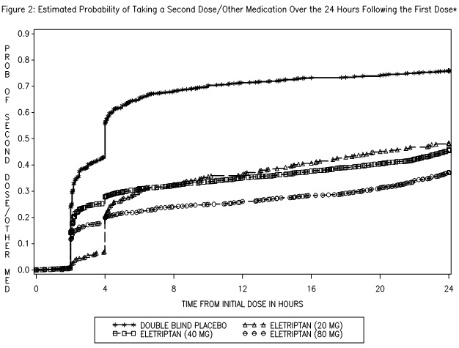
*This Kaplan-Meier plot is based on data obtained in 7 placebo-controlled trials in adults (Studies 1 through 7). Patients were instructed to take a second dose of study medication as follows: a) in the event of no response at 2 hours (studies 2 and 4-7) or at 4 hours (study 3); b) in the event of headache recurrence within 24 hours (studies 2-7). Patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. It should be noted that the protocols did not allow re-medication within 2 hours post dose.
The efficacy of RELPAX was unaffected by the duration of attack, gender or age of the patient, relationship to menses, or concomitant use of estrogen replacement therapy/oral contraceptives or frequently used migraine prophylactic drugs.
In a single study in adolescents (n=274), there were no statistically significant differences between treatment groups. The headache response rate at 2 hours was 57% for both RELPAX 40 mg Tablets and placebo.


If the migraine has not resolved by 2 hours after taking RELPAX, or returns after transient improvement, a second dose may be administered at least 2 hours after the first dose. The maximum daily dose should not exceed 80 mg.
The safety of treating an average of more than 3 migraine attacks in a 30-day period has not been established.
Pregnancy: Based on animal data, may cause fetal harm (
Available human data on the use of RELPAX in pregnant women are not sufficient to draw conclusions about drug-associated risk for major birth defects and miscarriage. In animal studies, oral administration of eletriptan during pregnancy or throughout pregnancy and lactation was associated with developmental toxicity (decreased fetal and pup weights, increased incidences of fetal structural abnormalities, decreased pup viability) at clinically-relevant doses
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The reported rate of major birth defects among deliveries to women with migraine ranged from 2.2% to 2.9% and the reported rate of miscarriage was 17%, which were similar to rates reported in women without migraine.
Several studies have suggested that women with migraine may be at increased risk of preeclampsia and gestational hypertension during pregnancy.
A study using linked data from the Medical Birth Registry of Norway to the Norwegian Prescription Database compared pregnancy outcomes in women who redeemed prescriptions for triptans during pregnancy, as well as a migraine disease comparison group who redeemed prescriptions for triptans before pregnancy only. Of the 189 women who redeemed prescriptions for eletriptan during the first trimester, 4 (2.1%) had infants with major congenital malformations, while for the 174 women who redeemed prescriptions for eletriptan before, but not during, pregnancy, 11 (6.3%) had infants with major congenital malformations. Methodological limitations of this study, including small size of the eletriptan population and infrequent events, do not allow for thorough characterization of risk.
When pregnant rats were administered eletriptan (0, 10, 30, or 100 mg/kg/day) during the period of organogenesis, fetal weights were decreased and the incidences of vertebral and sternebral variations were increased at 100 mg/kg/day (approximately 12 times the maximum recommended human dose [MRHD] of 80 mg/day on a mg/m2basis). The 30 and 100 mg/kg/day doses were also maternally toxic, as evidenced by decreased maternal body weight gain during gestation. The no-effect dose for adverse effects on embryofetal development in rats was 30 mg/kg/day, which is approximately 4 times the MRHD on a mg/m2basis.
When eletriptan (0, 5, 10, or 50 mg/kg/day) was orally administered to pregnant rabbits throughout organogenesis, fetal weights were decreased at 50 mg/kg/day. The incidences of fused sternebrae and vena cava deviations were increased at all doses. Maternal toxicity was not evident at any dose. A no-effect dose for adverse effects on embryofetal development in rabbits was not established; the lowest dose tested (5 mg/kg/day) is similar to the MRHD on a mg/m2basis.
Oral administration of eletriptan (0, 5, 15, or 50 mg/kg/day) to female rats throughout pregnancy and lactation resulted in a decrease in offspring viability and body weight at the highest dose tested. The no-effect dose for adverse effects on pre- and postnatal development in rats (15 mg/kg/day) is approximately 2 times the MRHD on a mg/m2basis.
RELPAX is contraindicated in patients with:
• Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal's angina[see].5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's AnginaRELPAX is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of RELPAX. Some of these reactions occurred in patients without known CAD. RELPAX may cause coronary artery vasospasm (Prinzmetal's angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving RELPAX. Do not use RELPAX if there is evidence of CAD or coronary artery vasospasm
[see Contraindications (4)].For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first RELPAX dose in a medically-supervised setting and performing an electrocardiogram (ECG) immediately following administration of RELPAX. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of RELPAX.• Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders[see].5.2 ArrhythmiasLife-threatening disturbances of cardiac rhythm including ventricular tachycardia and ventricular fibrillation leading to death have been reported within a few hours following the administration of 5-HT1agonists. Discontinue RELPAX if these disturbances occur. RELPAX is contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
[see Contraindications (4)].• History of stroke, transient ischemic attack (TIA), or history or current evidence of hemiplegic or basilar migraine because these patients are at a higher risk of stroke[see].5.4 Cerebrovascular EventsCerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not.
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with symptoms atypical of migraine, other potentially serious neurological conditions need to be excluded. RELPAX is contraindicated in patients with a history of stroke or TIA
[see Contraindications (4)].• Peripheral vascular disease[see].5.5 Other Vasospasm ReactionsRELPAX may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), and Raynaud's syndrome. In patients who experience symptoms or signs suggestive of a vasospastic reaction following the use of any 5-HT1agonist, rule out a vasospastic reaction before receiving additional RELPAX doses
[see Contraindications (4)].• Ischemic bowel disease[see].5.5 Other Vasospasm ReactionsRELPAX may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), and Raynaud's syndrome. In patients who experience symptoms or signs suggestive of a vasospastic reaction following the use of any 5-HT1agonist, rule out a vasospastic reaction before receiving additional RELPAX doses
[see Contraindications (4)].• Uncontrolled hypertension[see].5.8 Increase in Blood PressureSignificant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with RELPAX. RELPAX is contraindicated in patients with uncontrolled hypertension
[see Contraindications (4)].• Recent use (i.e., within 24 hours) of another 5-hydroxytryptamine1 (5-HT1) agonist, ergotamine-containing medication, or ergot-type medication such as dihydroergotamine (DHE) or methysergide[see.]7.1 Ergot-Containing Drugs Including Other 5-HT1B/1DAgonistsErgot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine [DHE] or methysergide) and RELPAX within 24 hours of each other is contraindicated
.Concomitant use of other 5-HT1agonists within 24 hours of RELPAX treatment is contraindicated[see Contraindications (4)].• Hypersensitivity to RELPAX (angioedema and anaphylaxis seen)[see].5.9 Anaphylactic/Anaphylactoid ReactionsThere have been reports of anaphylaxis, anaphylactoid, and hypersensitivity reactions including angioedema in patients receiving RELPAX. Such reactions can be life-threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. RELPAX is contraindicated in patients with a history of hypersensitivity reaction to RELPAX
[see Contraindications (4)].• Recent use (i.e., within at least 72 hours) of the following potent CYP3A4 inhibitors: ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir, or nelfinavir[seeand7.2 CYP3A4 InhibitorsPotent CYP3A4 inhibitors significantly increase the exposure of RELPAX. RELPAX should not be used within at least 72 hours of treatment with potent CYP3A4 inhibitors
[see Contraindications (4)and Clinical Pharmacology (12.3)].].12.3 PharmacokineticsAbsorptionEletriptan is well absorbed after oral administration with peak plasma levels occurring approximately 1.5 hours after dosing to healthy subjects. In patients with moderate to severe migraine, the median Tmaxis 2.0 hours. The mean absolute bioavailability of eletriptan is approximately 50%. The oral pharmacokinetics are slightly more than dose-proportional over the clinical dose range. The AUC and Cmaxof eletriptan are increased by approximately 20 to 30% following oral administration with a high fat meal. RELPAX can be taken with or without food.
DistributionThe volume of distribution of eletriptan following IV administration is 138L. Plasma protein binding is moderate and approximately 85%.
MetabolismThe N-demethylated metabolite of eletriptan is the only known active metabolite. This metabolite causes vasoconstriction similar to eletriptan in animal models. Though the half-life of the metabolite is estimated to be about 13 hours, the plasma concentration of the N-demethylated metabolite is 10-20% of parent drug and is unlikely to contribute significantly to the overall effect of the parent compound.
In vitrostudies indicate that eletriptan is primarily metabolized by cytochrome P-450 enzyme CYP3A4[see Contraindications (4)and Drug Interactions (7.2)].EliminationThe terminal elimination half-life of eletriptan is approximately 4 hours. Mean renal clearance (CLR) following oral administration is approximately 3.9 L/h. Non-renal clearance accounts for about 90% of the total clearance.
Specific PopulationsAgeThe pharmacokinetics of eletriptan are generally unaffected by age. Blood pressure was increased to a greater extent in elderly subjects than in young subjects
[see Use in Specific Populations (8.5)]. The pharmacokinetic disposition of eletriptan in the elderly is similar to that seen in younger adults.There is a statistically significant increased half-life (from about 4.4 hours to 5.7 hours) between elderly (65 to 93 years of age) and younger adult subjects (18 to 45 years of age)
[see Use in Specific Populations (8.5)].GenderThe pharmacokinetics of eletriptan are unaffected by gender.
RaceA comparison of pharmacokinetic studies run in western countries with those run in Japan has indicated an approximate 35% reduction in the exposure of eletriptan in Japanese male volunteers compared to western males. Population pharmacokinetic analysis of two clinical studies indicates no evidence of pharmacokinetic differences between Caucasians and non-Caucasian patients.
Menstrual CycleIn a study of 16 healthy females, the pharmacokinetics of eletriptan remained consistent throughout the phases of the menstrual cycle.
Renal ImpairmentThere was no significant change in clearance observed in subjects with mild, moderate or severe renal impairment, though blood pressure elevations were observed in this population
[see Warnings and Precautions (5.8)].Hepatic ImpairmentSubjects with mild or moderate hepatic impairment demonstrated an increase in both AUC (34%) and half-life. The Cmaxwas increased by 18%. No dose adjustment is necessary in subjects with mild or moderate hepatic impairment. The effects of severe hepatic impairment on eletriptan metabolism have not been evaluated
[see Use in Specific Populations (8.6)].Drug Interaction StudiesCYP3A4 InhibitorsIn vitrostudies have shown that eletriptan is metabolized by the CYP3A4 enzyme. A clinical study demonstrated about a 3-fold increase in Cmaxand about a 6-fold increase in the AUC of eletriptan when combined with ketoconazole. The half-life increased from 5 hours to 8 hours and the Tmaxincreased from 2.8 hours to 5.4 hours. Another clinical study demonstrated about a 2-fold increase in Cmaxand about a 4-fold increase in AUC when erythromycin was co-administered with eletriptan. It has also been shown that co-administration of verapamil and eletriptan yields about a 2-fold increase in Cmaxand about a 3-fold increase in AUC of eletriptan, and that co-administration of fluconazole and eletriptan yields about a 1.4-fold increase in Cmaxand about a 2-fold increase in AUC of eletriptan.RELPAX is contraindicated within at least 72 hours of treatment with the following potent CYP3A4 inhibitors: ketoconazole, itraconazole, nefazodone, troleandomycin, clarithromycin, ritonavir and nelfinavir. RELPAX should not be used within 72 hours with drugs that have demonstrated potent CYP3A4 inhibition
[see Contraindications (4)].PropranololThe Cmaxand AUC of eletriptan were increased by 10 and 33%, respectively, in the presence of propranolol. No interactive increases in blood pressure were observed. No dosage adjustment appears to be needed for patients taking propranolol.
The Effect of Eletriptan on Other DrugsThe effect of eletriptan on enzymes other than cytochrome P450 has not been investigated.
In vitrohuman liver microsome studies suggest that eletriptan has little potential to inhibit CYP1A2, 2C9, 2E1 and 3A4 at concentrations up to 100 µM. While eletriptan has an effect on CYP2D6 at high concentration, this effect should not interfere with metabolism of other drugs when eletriptan is used at recommended doses. There is noin vitroorin vivoevidence that clinical doses of eletriptan will induce drug metabolizing enzymes. Therefore, eletriptan is unlikely to cause clinically important drug interactions mediated by these enzymes.
RELPAX should only be used where a clear diagnosis of migraine has been established.