Rethymic
(Allogenic Thymocyte-Depleted Thymus Tissue-Agdc)Rethymic Prescribing Information
RETHYMIC® is indicated for immune reconstitution in pediatric patients with congenital athymia.
RETHYMIC is administered by a surgical procedure. The recommended dose range is 5,000 to 22,000 mm2 of RETHYMIC/m2 recipient body surface area (BSA). (2) Immunosuppressive therapy is recommended for patients receiving RETHYMIC based on disease phenotype and PHA levels. (
The efficacy of RETHYMIC was evaluated in 10 prospective, single-center, open-label studies that enrolled a total of 105 patients, including 95 patients in the primary efficacy analysis. The demographics and baseline characteristics of the patients enrolled in the clinical studies were similar across studies. Across the efficacy population, 59% were male; 70% were White, 22% were Black, 4% were Asian/Pacific Islander; 2% were American Indian/Alaskan Native; and 2% were multi-race. The median (range) age at the time of treatment was 9 months (1-36). The diagnosis of congenital athymia was based on flow cytometry documenting fewer than 50 naïve T cells/mm3(CD45RA+, CD62L+) in the peripheral blood or less than 5% of total T cells being naïve in phenotype in 91/95 patients (range 0-98 naïve T cells/mm3). In addition to congenital athymia, patients also had complete DiGeorge syndrome (cDGS; also referred to as complete DiGeorge anomaly (cDGA)) if they also met at least one of the following criteria: congenital heart defect, hypoparathyroidism (or hypocalcemia requiring calcium replacement), 22q11 hemizygosity, 10p13 hemizygosity, CHARGE (coloboma, heart defect, choanal atresia, growth and development retardation, genital hypoplasia, ear defects including deafness) syndrome, or CHD7 mutation. Across the efficacy population, 93 patients (98%) were diagnosed with cDGS, and the most common DiGeorge gene mutations or syndromic associations were Chromosome 22q11.2 deletion (36 patients; 38%) and CHARGE syndrome (23 patients; 24%). There were 35 patients with missing or no identified genetic mutations. Two (2%) patients had FOXN1 deficiency, and 1 patient (1%) had a TBX variant. There were 50 (53%) patients with typical cDGS; these patients had congenital athymia with the absence of a T cell-related rash. There were 42 (44%) patients diagnosed with atypical cDGS; these patients may have had a rash, lymphadenopathy, or oligoclonal T cells. Patients who did not have congenital athymia (e.g. SCID) and patients with prior transplants, including thymus and HCT, were excluded from the efficacy analysis population. The baseline demographics and disease characteristics were similar in the safety population.
Patients with heart surgery anticipated within 4 weeks prior to, or 3 months after, the planned RETHYMIC treatment date, patients with human immunodeficiency virus (HIV) infection, and patients who were not considered good surgical candidates were excluded from study participation.
Patients in the efficacy population received RETHYMIC in a single surgical procedure at a dose of 4,900 to 24,000 mm2of RETHYMIC / recipient BSA in m2. Patients were assigned to receive immunosuppressive therapy prior to and/or after treatment according to their disease phenotype and pre-RETHYMIC PHA response. Table 2 summarizes the criteria used to administer immunosuppression. Table 3 summarizes the specific immunosuppressant dosing used in RETHYMIC clinical studies. No patients were retreated with RETHYMIC.
| Complete DiGeorge Anomaly Phenotype | Phytohemagglutinin (PHA) ResponseValues for PHA response are reported from Duke University Medical Center and may not be comparable to values reported at other clinical laboratories. A patient background value (cells without stimulus) of less than 5,000 cpm was required to consider PHA test results valid. A normal control value of > 75,000 cpm was also required during clinical studies. | Immunosuppression Used During Clinical Studies with RETHYMIC |
|---|---|---|
| Abbreviations: ATG-R: anti-thymocyte globulin [rabbit] (Thymoglobulin); cpm: counts per minute; MMF: mycophenylate mofetil; PHA: phytohemagglutinin | ||
| Typical | < 5,000 cpm or < 20-fold response to PHA over background | None |
| Typical | ≥ 5,000 cpm and < 50,000 cpm or Evidence of maternal engraftment |
|
| Typical | ≥ 50,000 cpm |
|
| Atypical | < 40,000 cpm on immunosuppression or < 75,000 cpm when not on immunosuppression |
|
| Atypical | ≥ 40,000 cpm on immunosuppression or ≥ 75,000 cpm when not on immunosuppression or Evidence of maternal engraftment |
|
| Immunosuppressant | Dose of Immunosuppressant |
|---|---|
| Abbreviations: ATG-R: anti-thymocyte globulin [rabbit] (Thymoglobulin); IV: intravenous; MMF: mycophenylate mofetil; PO: oral | |
| ATG-R |
|
| MethylprednisoloneAdditional pre-implantation corticosteroids (methylprednisolone) were used for atypical patients if pre-implantation CD3 + T cell numbers or the absolute lymphocyte count (ALC) was greater than 4,000 cells/mm 3. A starting dose of 1 mg/kg/day was used if the T cell count or ALC was between 4,000 and 10,000 cells/mm 3. A dose of 2 mg/kg/day was used if the T cell count was > 10,000 cells/mm 3.,Corticosteroids (methylprednisolone or prednisolone) were initiated as soon as the diagnosis was confirmed in patients with evidence of maternal engraftment or with atypical cDGS and a PHA response of > 40,000 cpm on immunosuppression or > 75,000 cpm when not on immunosuppression. The steroid was weaned as soon as possible when the rash and other symptoms were brought under control. |
|
| CyclosporineCyclosporine was initiated as soon as the diagnosis was confirmed and at least 7 days prior to ATG-R administration. If the CD3 + T cells fell and remained below 50/mm 3, cyclosporine was weaned to have a cyclosporine trough level of 100 to 150 ng/mL. If the T cell count remained over 50/mm 3, cyclosporine was maintained until the naive T cells were 10% of CD3 + T cells. Cyclosporine was then weaned over 10 weeks. To preserve renal function, the initiation of cyclosporine may have been delayed prior to implantation. Renal function was monitored according to the cyclosporine or tacrolimus prescribing information.,A higher target trough concentration of 250 to 300 ng/mL was used in patients with evidence of maternal engraftment or with atypical cDGS and a PHA response of > 40,000 cpm on immunosuppression or > 75,000 cpm when not on immunosuppression.,If the patient could not tolerate cyclosporine due to adverse events (AEs), then the immunosuppression could have been changed to tacrolimus (target trough concentration of 7 to 10 ng/mL). In patients with evidence of maternal engraftment or with atypical cDGS and a PHA response of > 40,000 cpm on immunosuppression or > 75,000 cpm when not on immunosuppression, the tacrolimus target trough level was 10 to 15 ng/mL. |
|
| Basiliximab |
|
| MMF |
|
| AlemtuzumabPremedications given 30 minutes prior to alemtuzumab include methylprednisolone (1 mg/kg IV), acetaminophen (10 mg/kg IV), and diphenhydramine, (0.5 mg/kg IV). |
|
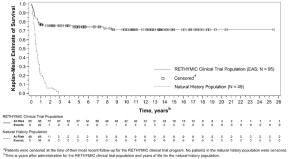
The Kaplan-Meier estimated survival rates were 77% (95% CI [0.670, 0.841]) at 1 year and 76% (95% CI [0.658, 0.832]) at 2 years. For patients who were alive at 1 year after treatment with RETHYMIC, the survival rate was 94% at a median follow-up of 10.7 years.
Without treatment, congenital athymia is fatal in childhood. In a natural history population observed from 1991 through 2017, 49 patients diagnosed with congenital athymia received supportive care only. The 2-year survival rate was 6%, with all patients dying by 3 years of age. This population included 33 (67%) males. The most common cause of death was infection in 26 (53%) patients. Other common causes (≥10%) included support withdrawn in 7 (14%) patients, respiratory arrest in 5 (10%) patients, and cardiac arrest in 5 (10%) patients.
The Kaplan-Meier estimated survival rates for the RETHYMIC clinical trial population and the natural history population are shown in Figure 5. Four patients with >50 naïve T cells/mm3(CD45RA+, CD62L+) at time of RETHYMIC administration have been treated; 2 (50%) were alive with follow-up less than 2 years.
RETHYMIC significantly reduced the number of infections over time. In the first year after treatment with RETHYMIC, the number of patients with an infection event onset 6 to ≤ 12 months after treatment decreased by 38% (from 63 to 39) relative to the number of patients with an infection event onset in the first 6 months post-treatment. A two-year analysis showed a decrease in both the number of patients with an infection event and the mean number of infection events per patient, with an onset in the first 12 months post-treatment as compared to 12 to ≤ 24 months after treatment. There was a mean difference of 2.9 events (p<0.001) per patient.
Naïve CD4+and CD8+T cells reconstituted over the first year, with a durable increase through Year 2. Median (minimum, maximum) naïve CD4+T cells/mm3increased from a baseline of 1 (0, 38) to values of 42 (0, 653), 212 (1, 751), and 275 (33, 858) at 6, 12, and 24 months after treatment with RETHYMIC, respectively. Median naïve CD8+T cells/mm3increased from a baseline of 0 (0, 46) to values of 9 (0, 163), 58 (0, 304), and 86 (6, 275) at 6, 12, and 24 months after treatment with RETHYMIC, respectively. This was accompanied by functional improvements based on T cell proliferative responses to PHA.

RETHYMIC consists of yellow to brown slices of processed thymus tissue with varying thickness and shape. Each drug product dish contains up to 4 RETHYMIC slices that adhere to circular filter membranes on top of surgical sponges in 5 mL of medium. The RETHYMIC slices are variable in size and shape; a RETHYMIC slice is defined as the contents of a single filter membrane. The dosage is based on the total surface area of the RETHYMIC slices, and the amount administered is calculated based on recipient BSA. The surgeon should implant as many RETHYMIC slices as possible within the recommended dose range of 5,000 to 22,000 mm2 of RETHYMIC/m2 recipient BSA. The manufacturer calculates the dose in advance for the specific patient; the amount of product provided is adjusted at the manufacturing facility to ensure the maximum dose for the patient cannot be exceeded. Up to 42 RETHYMIC slices will be provided for each patient. At the time of surgery, the manufacturing personnel will inform the surgical team of the portion of the product that represents the minimum dose.
There are no clinical data with RETHYMIC in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with RETHYMIC. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
None.
- Immune reconstitution sufficient to protect from infection is unlikely to develop prior to 6 to 12 months after treatment with RETHYMIC. Given the immunocompromised condition of athymic patients, infection control measures should be followed until the development of thymic function can be established. ( )
5.1 Infection Control and ImmunoprophylaxisImmune reconstitution sufficient to protect from infection is unlikely to develop prior to 6-12 months after treatment with RETHYMIC. Given the immunocompromised condition of athymic patients, follow infection control measures until the development of thymic function is established as measured through flow cytometry. This should include counseling patients and their caregivers on good handwashing practices and minimizing exposure to visitors. Monitor patients closely for signs of infection, including fever. If a fever develops, assess the patient by blood and other cultures and treat with antimicrobials as clinically indicated.
Patients should be maintained on immunoglobulin replacement therapy until all of the following criteria are met:
- No longer on immunosuppression (at least 10% of CD3+T cells are naïve in phenotype).
- At least 9 months post-treatment.
- Phytohemagglutinin (PHA) response within normal limits.
- Normal serum IgA is also desirable but not required.
Two months after stopping immunoglobulin replacement therapy, the IgG trough level should be checked.
- If the IgG trough level is in the normal range for age, the patient can remain off of immunoglobulin replacement.
- If the IgG trough level is lower than the normal range for age, immunoglobulin replacement therapy should be restarted and continued for a year before being retested using the above guidelines.
Prior to and after treatment with RETHYMIC, patients should be maintained on Pneumocystis jiroveci pneumonia prophylaxis until all of the following criteria are met:
- No longer on immunosuppression (at least 10% of CD3+ T cells are naïve in phenotype).
- At least 9 months post-treatment.
- PHA response within normal limits.
- CD4+ T cell count > 200 cells/mm3.
- Monitor and treat patients at risk for the development of graft versus host disease (GVHD). ( )
5.2 Graft versus Host DiseaseIn clinical studies with RETHYMIC, GVHD occurred in 11 (10%) RETHYMIC-treated patients of whom 6 (55%) died. RETHYMIC may cause or exacerbate pre-existing GVHD. Seven patients (7%) experienced autologous GVHD, 3 patients (3%) experienced GVHD due to maternal cells and 1 patient (1%) experienced GVHD due to cells from a prior hematopoietic cell transplant (HCT). Risk factors for GVHD include atypical complete DiGeorge anomaly phenotype, prior HCT and maternal engraftment. GVHD may manifest as fever, rash, lymphadenopathy, elevated bilirubin and liver enzymes, enteritis, and/or diarrhea. Patients with elevated baseline T cell proliferative response to PHA > 5,000 cpm or > 20-fold over background should receive immunosuppressive therapies to decrease the risk of GVHD (Table 2 and Table 3). Development of GVHD symptoms should be closely monitored and promptly treated.
- Monitor for the development of autoimmune disorders, including complete blood counts with differential, liver enzymes, serum creatinine, urinalysis, and thyroid function. ( )
5.3 Autoimmune DisordersThirty-seven patients (35%) in the RETHYMIC clinical program experienced autoimmune-related adverse reactions. These events included: thrombocytopenia (including idiopathic thrombocytopenic purpura) in 13 patients (12%), neutropenia in 9 patients (9%), proteinuria in 7 patients (7%), hemolytic anemia in 7 patients (7%), alopecia in 4 patients (4%), hypothyroidism in 2 patients (2%), autoimmune hepatitis in 2 patients (2%), and autoimmune arthritis (juvenile idiopathic and psoriatic arthritis) in 2 patients (2%). One patient (1%) each experienced transverse myelitis, albinism, hyperthyroidism, and ovarian failure. The onset of autoimmune related events ranged from the three days before the surgical implantation procedure until 16 years post-treatment. Most events occurred within the first year after treatment.
Monitor complete blood counts with differential weekly for the first 2 months post-treatment and then monthly through 12 months post-treatment. Liver enzymes including aspartate aminotransferase and alanine aminotransferase, serum creatinine levels, and urinalysis should be performed monthly for 3 months and then every 3 months through 12 months post-treatment. Thyroid function studies should be performed prior to treatment and then at 6 months and 12 months post-treatment. After 12 months, testing should be performed annually.
- Pre-existing renal impairment is a risk factor for death. ( )
5.4 Renal ImpairmentTen patients with renal impairment (elevated serum creatinine at baseline) were treated in studies with RETHYMIC. Five of these patients died within 1 year and a sixth patient died 3 years after treatment with RETHYMIC. Renal impairment at baseline is considered a risk factor for death.
- Pre-existing cytomegalovirus infection may result in death prior to the development of thymic function. ( )
5.5 Cytomegalovirus InfectionIn clinical studies with RETHYMIC, 4 out of 4 patients with preexisting CMV infection prior to treatment with RETHYMIC died. The benefits/risks of treatment should be considered prior to treating patients with pre-existing CMV infection.
- Monitor for the development of lymphoproliferative disorder (blood cancer). ( )
5.6 MalignancyBecause of the underlying immune deficiency, patients who receive RETHYMIC may be at risk of developing post-treatment lymphoproliferative disorder (blood cancer). The infant tissue donor is screened for Epstein-Barr virus (EBV) and cytomegalovirus (CMV), but patients should be tested for EBV and CMV using PCR prior to and 3 months following treatment with RETHYMIC, or after any exposure to or suspected infection with CMV or EBV.
- Transmission of infectious diseases may occur because RETHYMIC is derived from human tissue. ( )
5.7 Transmission of Serious Infections and Transmissible Infectious DiseasesTransmission of infectious disease may occur because RETHYMIC is derived from human tissue. Disease may be caused by known or unknown infectious agents. Donors are screened for increased risk of infection with human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), hepatitis B virus (HBV), hepatitis C virus (HCV),
Treponema pallidum, Trypanosoma cruzi, West Nile virus (WNV), transmissible spongiform encephalopathy (TSE) agents, vaccinia and Zika virus. Donors are also screened for clinical evidence of sepsis, and communicable disease risks associated with xenotransplantation. Blood samples (from the infant tissue donor or the birth mother, as applicable) are tested for HIV types 1, 2, and O, HTLV types I and II, HBV, HCV,T. pallidum, WNV, andT. cruzi. Blood from the infant tissue donor is also tested forToxoplasma gondii, Epstein-Barr virus (EBV) and CMV. RETHYMIC is tested for sterility, endotoxin, and mycoplasma. These measures do not eliminate the risk of transmitting these or other infectious diseases and disease agents.Testing of maternal and infant donor blood is also performed for evidence of donor infection due to cytomegalovirus (CMV).
Product manufacturing includes porcine- and bovine-derived reagents. While all animal-derived reagents are tested for animal viruses, bacteria, fungi, and mycoplasma before use, these measures do not eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents.
Final sterility and mycoplasma test results are not available at the time of use, but manufacturing personnel will communicate any positive results from sterility testing to the physician. Report the occurrence of transmitted infection to Sumitomo Pharma America at 833-369-9868.
- Immunizations should not be administered in patients who have received RETHYMIC until immune-function criteria have been met. ( )
5.8 Vaccine AdministrationImmunizations should not be administered in patients who have received RETHYMIC until immune-function criteria have been met.
Inactivated vaccines:Inactivated vaccines may be administered once all of the following criteria are met:
- Immunosuppressive therapies have been discontinued.
- Immunoglobulin (IgG) replacement therapy has been discontinued.
- The total CD4+T cell count is > 200 cells/mm3and there are more CD4+T cells than CD8+T cells (CD4+> CD8+).
It is recommended that no more than 2 inactivated vaccines be given per month.
Live Vaccines:Live virus vaccines should not be administered until patients have met the criteria for inactivated vaccines and received vaccinations with inactivated agents (e.g., tetanus toxoid). No additional vaccines (live or inactivated), except the inactivated influenza vaccine, should be given within 6 months after vaccination with a measles-containing vaccine or within 2 months after the varicella vaccine. Consider verifying response to vaccination with appropriate testing, in particular varicella and measles.
- Patients should be tested for anti-HLA antibodies prior to treatment. ( )
5.9 Anti-HLA AntibodiesAll patients should be screened for anti-HLA antibodies prior to receiving RETHYMIC. Patients testing positive for anti-HLA antibodies should receive RETHYMIC from a donor who does not express those HLA alleles.