Rifabutin
Rifabutin Prescribing Information
Rifabutin capsules are indicated for the prevention of disseminated
It is recommended that rifabutin capsules be administered at a dose of 300 mg once daily. For those patients with propensity to nausea, vomiting, or other gastrointestinal upset, administration of rifabutin at doses of 150 mg twice daily taken with food may be useful. Doses of rifabutin may be administered mixed with foods such as applesauce.
For patients with severe renal impairment (creatinine clearance less than 30 mL/min), consider reducing the dose of rifabutin by 50%, if toxicity is suspected. No dosage adjustment is required for patients with mild to moderate renal impairment. Reduction of the dose of rifabutin may also be needed for patients receiving concomitant treatment with certain other drugs (see
Mild hepatic impairment does not require a dose modification. The pharmacokinetics of rifabutin in patients with moderate and severe hepatic impairment is not known.
Rifabutin capsules are contraindicated in patients who have had clinically significant hypersensitivity to rifabutin or to any other rifamycins.
Rifabutin capsules are contraindicated in patients being treated with cabotegravir/rilpivirine prolonged-release injectable suspension (see
Rifabutin capsules were generally well tolerated in the controlled clinical trials. Discontinuation of therapy due to an adverse event was required in 16% of patients receiving rifabutin, compared to 8% of patients receiving placebo in these trials. Primary reasons for discontinuation of rifabutin were rash (4% of treated patients), gastrointestinal intolerance (3%), and neutropenia (2%).
The following table enumerates adverse experiences that occurred at a frequency of 1% or greater, among the patients treated with rifabutin in studies 023 and 027.
Adverse event | Rifabutin (n = 566) % | Placebo (n = 580) % |
Body as a whole | ||
| Abdominal pain | 4 | 3 |
| Asthenia | 1 | 1 |
| Chest pain | 1 | 1 |
| Fever | 2 | 1 |
| Headache | 3 | 5 |
| Pain | 1 | 2 |
Blood and lymphatic system | ||
| Leucopenia | 10 | 7 |
| Anemia | 1 | 2 |
Digestive System | ||
| Anorexia | 2 | 2 |
| Diarrhea | 3 | 3 |
| Dyspepsia | 3 | 1 |
| Eructation | 3 | 1 |
| Flatulence | 2 | 1 |
| Nausea | 6 | 5 |
| Nausea and vomiting | 3 | 2 |
| Vomiting | 1 | 1 |
Musculoskeletal system | ||
| Myalgia | 2 | 1 |
Nervous system | ||
| Insomnia | 1 | 1 |
Skin and appendages | ||
| Rash | 11 | 8 |
Special senses | ||
| Taste perversion | 3 | 1 |
Urogenital system | ||
| Discolored urine | 30 | 6 |
Considering data from the 023 and 027 pivotal trials, and from other clinical studies, rifabutin appears to be a likely cause of the following adverse events which occurred in less than 1% of treated patients: flu-like syndrome, hepatitis, hemolysis, arthralgia, myositis, chest pressure or pain with dyspnea, skin discoloration, thrombocytopenia, pancytopenia and jaundice.
The following adverse events have occurred in more than one patient receiving rifabutin, but an etiologic role has not been established: seizure, paresthesia, aphasia, confusion, and non-specific T wave changes on electrocardiogram.
When rifabutin was administered at doses from 1050 mg/day to 2400 mg/day, generalized arthralgia and uveitis were reported. These adverse experiences abated when rifabutin was discontinued.
Mild to severe, reversible uveitis has been reported less frequently when rifabutin is used at 300 mg as monotherapy in MAC prophylaxis versus rifabutin in combination with clarithromycin for MAC treatment (see also
Uveitis has been infrequently reported when rifabutin is used at 300 mg/day as monotherapy in MAC prophylaxis of HIV-infected persons, even with the concomitant use of fluconazole and/or macrolide antibacterials. However, if higher doses of rifabutin are administered in combination with these agents, the incidence of uveitis is higher.
Patients who developed uveitis had mild to severe symptoms that resolved after treatment with corticosteroids and/or mydriatic eye drops; in some severe cases, however, resolution of symptoms occurred after several weeks.
When uveitis occurs, temporary discontinuance of rifabutin and ophthalmologic evaluation are recommended. In most mild cases, rifabutin may be restarted; however, if signs or symptoms recur, use of rifabutin should be discontinued (Morbidity and Mortality Weekly Report, September 9, 1994).
Corneal deposits have been reported during routine ophthalmologic surveillance of some HIV-positive pediatric patients receiving rifabutin as part of a multiple drug regimen for MAC prophylaxis. The deposits are tiny, almost transparent, asymptomatic peripheral and central corneal deposits, and do not impair vision.
The following table enumerates the changes in laboratory values that were considered as laboratory abnormalities in Studies 023 and 027.
Laboratory abnormalities | Rifabutin (n = 566) % | PLACEBO (n = 580) % |
Chemistry | ||
| Increased alkaline phosphatase1 | <1 | 3 |
| Increased SGOT2 | 7 | 12 |
| Increased SGPT2 | 9 | 11 |
Hematology | ||
| Anemia3 | 6 | 7 |
| Eosinophilia | 1 | 1 |
| Leukopenia4 | 17 | 16 |
| Neutropenia5 | 25 | 20 |
| Thrombocytopenia6 | 5 | 4 |
Includes grades 3 or 4 toxicities as specified:
1 All values >450 U/L
2 All values >150 U/L
3 All hemoglobin values <8.0 g/dL
4 All WBC values <1,500/mm3
5 All ANC values <750/mm3
6 All platelet count values <50,000/mm3
The incidence of neutropenia in patients treated with rifabutin was significantly greater than in patients treated with placebo (p = 0.03). Although thrombocytopenia was not significantly more common among patients treated with rifabutin in these trials, rifabutin has been clearly linked to thrombocytopenia in rare cases. One patient in Study 023 developed thrombotic thrombocytopenic purpura, which was attributed to rifabutin.
Adverse reactions identified through post-marketing surveillance by system organ class (SOC) are listed below:
Pyrexia, rash and other hypersensitivity reactions such as eosinophilia and bronchospasm might occur, as has been seen with other antibacterials.
A limited occurrence of skin discoloration has been reported.
Rifabutin has been associated with the occurrence of DRESS as well as other SCARs such as SJS, TEN, and AGEP (see
Hypersensitivity to rifamycins have been reported including flu-like symptoms, bronchospasm, hypotension, urticaria, angioedema, conjunctivitis, thrombocytopenia or neutropenia.
Rifabutin induces CYP3A enzymes and therefore may reduce the plasma concentrations of drugs metabolized by those enzymes. This effect may reduce the efficacy of standard doses of such drugs, which include itraconazole, clarithromycin, and saquinavir.
Some drugs that inhibit CYP3A may significantly increase the plasma concentration of rifabutin. Therefore, carefully monitor for rifabutin associated adverse events in those patients also receiving CYP3A inhibitors, which include fluconazole and clarithromycin. In some cases, the dosage of rifabutin may need to be reduced when it is co-administered with CYP3A inhibitors.
Table 2 summarizes the results and magnitude of the pertinent drug interactions assessed with rifabutin. The clinical relevance of these interactions and subsequent dose modifications should be judged in light of the population studied, severity of the disease, patient's drug profile, and the likely impact on the risk/benefit ratio.
Co-administered drug | Dosing regimen of co-administered drug | Dosing regimen of rifabutin | Study population (n) | Effect on rifabutin | Effect on co-administered drug | Recommendation |
ANTIRETROVIRALS | ||||||
| Amprenavir | 1200 mg twice a day for 10 days | 300 mg once a day for 10 days | Healthy male subjects (6) | ↑ AUC by 193%, ↑ Cmax by 119% | Reduce rifabutin dose by at least 50%. Monitor closely for adverse reactions. | |
| Atazanavir/ Ritonavir | 300/100 mg once daily | 150 mg twice weekly | Healthy adult subjects | 48% ↑ in AUC, 149% ↑ Cmax of rifabutin. 990% ↑ in AUC, 677%↑ Cmax of25-O-desacetyl-rifabutin. | No significant change in pharmacokinetics | A reduction in the dose of rifabutin (to 150 mg every other day or 3 times a week) is recommended. Increased monitoring for adverse reactions is warranted. |
| Bictegravir | 75 mg once a day | 300 mg once a day (fasted) | Healthy subjects | ND | ↓ AUC 38% ↓ Cmin 56% ↓ Cmax 20% | Co-administration of rifabutin with Biktarvy (bictegravir/ emtricitabine/tenofovir alafenamide) is not recommended due to an expected decrease in tenofovir alafenamide in addition to the reported reduction in bictegravir. Refer to Biktarvy prescribing information for additional information. |
| Darunavir/ Ritonavir | 600/100 mg twice a day for 12 days | 150 mg every other day for 12 days | Healthy HIV negative adults | No significant change in rifabutin pharmacokinetics. 881% ↑in AUC, 377% ↑ Cmax of 25-O-desacetyl-rifabutin | 57% ↑in AUC, 42% ↑ Cmax of darunavir. 66% ↑in AUC,68% ↑ Cmax of ritonavir. | A reduction in the dose of rifabutin (to 150 mg every other day or 3 times a week) is recommended. Increased monitoring for adverse reactions is warranted. |
| Delavirdine | 400 mg three times a day | 300 mg once a day | HIV infected patients (7) | ↑ AUC by 230%, ↑ Cmax by 128% | ↓ AUC by 80%, ↓ Cmax by 75%, ↓ Cmin by 17% | CONTRAINDICATED |
| Didanosine | 167 or 250 mg twice a day for 12 days | 300 or 600 mg once a day for 12 days | HIV infected patients (11) | | ||
| Dolutegravir | 50 mg daily for 14 days | 300 mg daily for 14 days | Healthy adult subjects | ND | No significant change in dolutegravir pharmacokinetic s at steady state. | |
| Doravirine | 100 mg single dose | 300 mg once a day for 16 days | Healthy subjects (12) | ND | ↓ 50% in AUC, ↓ 68% in C24 ↔ in Cmax | If concomitant use is necessary, increase the doravirine dosage as instructed in doravirine-containing product prescribing information. |
| Elvitegravir/ Cobicistat | 150/50 mg daily | 300 mg daily or 150 mg every other day | Healthy subjects (12) | No significant change in rifabutin pharmacokinetics. 6.3-fold ↑in AUC, 4.8-fold ↑ Cmax of 25-O-desacetyl-rifabutin | No change in elvitegravir except 67% ↓ Ctrough of elvitegravir No change in cobicistat exposure. | Co-administration of rifabutin with elvitegravir/ cobicistat is not recommended due to an expected decrease in elvitegravir exposure. |
| Etravirine | 800 mg twice daily for 21 days | 300 mg daily on days 8 to 21 | Healthy volunteers (18) | No significant change in rifabutin pharmacokinetics. | 37% ↓ in AUC, 37% ↓ in Cmax and 35% ↓ in Cmin | No dose adjustment of rifabutin is required when etravirine is not co-administered with protease inhibitor/ritonavir. Rifabutin should not be co-administered with etravirine and boosted PIs due to potential for decreased effectiveness of etravirine. |
| Fosamprenavir/ ritonavir | 700 mg twice a day plus ritonavir 100 mg twice a day for 2 weeks | 150 mg every other day for 2 weeks | Healthy subjects (15) | ↔ AUCa ↓ Cmax by 15% | ↑ AUC by 35%b, ↑ Cmax by 36%, ↑ Cmin by 36%, | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with fosamprenavir/ritonavir combination. |
| Indinavir | 800 mg three times a day for 10 days | 300 mg once a day for 10 days | Healthy subjects (10) | ↑ AUC by 173%, ↑ Cmax by 134% | ↓ AUC by 34%, ↓ Cmax by 25%, ↓ Cmin by 39% | Reduce rifabutin dose by 50%, and increase indinavir dose from 800 mg to 1000 mg three times a day. |
| Lopinavir/ ritonavir | 400/100 mg twice a day for 20 days | 150 mg once a day for 10 days | Healthy subjects (14) | ↑ AUC by 203%c ↓ Cmax by 112% | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with lopinavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. | |
| Saquinavir/ ritonavir | 1000/100 mg twice a day for 14 or 22 days | 150 mg every 3 days for 21-22 days | Healthy subjects | ↑ AUC by 53%d ↑ Cmax by 88% (n=11) | ↓ AUC by 13%, ↓ Cmax by 15%, (n=19) | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with saquinavir/ritonavir combination. Monitor closely for adverse reactions. |
| Rilpivirine | 25 mg once a day | 300 mg once a day | Healthy subjects (18) | ND | ↓ AUC by 42% ↓ Cmin by 48% ↓ Cmax by 31% | Co-administration of rifabutin with Odefsey (rilpivirine/ tenofovir alafenamide/ emtricitabine) is not recommended, due to an expected decrease in tenofovir alafenamide in addition to the reported reduction in rilpivirine. Refer to Odefsey prescribing information for additional information. Co-administration of rifabutin with cabotegravir/rilpivirine prolonged-release injectable suspension is contraindicated |
| Ritonavir | 500 mg twice a day for 10 days | 150 mg once a day for 16 days | Healthy subjects (5) | ↑ AUC by 300%, ↑ Cmax by 150% | ND | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with lopinavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. |
| Tipranavir/ ritonavir | 500/200 twice a day for 15 doses | 150 mg single dose | Healthy subjects (20) | ↑ AUC by 190%, ↑ Cmax by 70% | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with tipranavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. | |
| Nelfinavir | 1250 mg twice a day for 7-8 days | 150 mg once a day for 8 days | HIV infected patients (11) | ↑ AUC by 83%,e ↑ Cmax by 19% | Reduce rifabutin dose by 50% (to 150 mg once a day) and increase the nelfinavir dose to 1250 mg twice a day. | |
| Zidovudine | 100 or 200 mg every four hours | 300 or 450 mg once a day | HIV infected patients (16) | ↓ AUC by 32%, ↓ Cmax by 48%, | Because zidovudine levels remained within the therapeutic range during coadministration of rifabutin, dosage adjustments are not necessary. | |
ANTI-HCV DRUGS | ||||||
| Sofosbuvir | 400 mg on day 1 and day 21 | 300 mg daily on day 10 to day 29 | Healthy subjects (20) | ND | 36% ↓ in Cmax and 24% ↓ AUC | Co-administration of rifabutin with sofosbuvir (alone or in combination) is not recommended. |
ANTIFUNGALS | ||||||
| Fluconazole | 200 mg once a day for 2 weeks | 300 mg once a day for 2 weeks | HIV infected patients (12) | ↑ AUC by 82%, ↑ Cmax by 88% | Monitor for rifabutin associated adverse events. Reduce rifabutin dose or suspend rifabutin use if toxicity is suspected. | |
| Posaconazole | 200 mg once a day for 10 days | 300 mg once a day for 17 days | Healthy subjects (8) | ↑ AUC by 72%, ↑ Cmax by 31% | ↓ AUC by 49%, ↓ Cmax by 43% | If co-administration of these two drugs cannot be avoided, patients should be monitored for adverse events associated with rifabutin administration, and lack of posaconazole efficacy. |
| Itraconazole | 200 mg once a day | 300 mg once a day | HIV Infected patients (6) | ↑f | ↓ AUC by 70%, ↓ Cmax by 75%, | If co-administration of these two drugs cannot be avoided, patients should be monitored for adverse events associated with rifabutin administration, and lack of itraconazole efficacy. In a separate study, one case of uveitis was associated with increased serum rifabutin levels following co-administration of rifabutin (300 mg once a day) with itraconazole (600-900 mg once a day). |
| Voriconazole | 400 mg twice a day for 7 days (maintenance dose) | 300 mg once a day for 7 days | Healthy male subjects (12) | ↑ AUC by 331%, ↑ Cmax by 195% | ↑ AUC by ~100%, ↑ Cmax by ~100%g | CONTRAINDICATED |
ANTI-PCP (Pneumocystis carinii pneumonia) | ||||||
| Dapsone | 50 mg once a day | 300 mg once a day | HIV infected patients (16) | ND | ↓ AUC by 27 -40% | |
| Sulfamethoxazole-Trimethoprim | 800/160 mg | 300 mg once a day | HIV infected patients (12) | ↓ AUC by 15-20% | | |
ANTI-MAC (Mycobacterium avium intracellulare complex) | ||||||
| Azithromycin | 500 mg once a day for 1 day, then 250 mg once a day for 9 days | 300 mg once a day | Healthy subjects (6) | | ||
| Clarithromycin | 500 mg twice a day | 300 mg once a day | HIV infected patients (12) | ↑ AUC by 75% | ↓ AUC by 50% | Monitor for rifabutin associated adverse events. Reduce dose or suspend use of rifabutin if toxicity is suspected. Alternative treatment for clarithromycin should be considered when treating patients receiving rifabutin |
ANTI-TB (Tuberculosis) | ||||||
| Ethambutol | 1200 mg | 300 mg once a day for 7 days | Healthy subjects (10) | ND | | |
| Isoniazid | 300 mg | 300 mg once a day for 7 days | Healthy subjects (6) | ND | | |
| Bedaquiline | 400 mg daily on day 1 and day 29 | 300 mg daily | Healthy subjects (17) | ND | No change in bedaquiline pharmacokinetics. 1.4-fold ↑ in M2 and approximately 3.0-fold ↑ in M3 metabolites of bedaquiline. | Avoid bedaquiline co-administration with rifabutin due to the adverse reactions associated with increased bedaquiline metabolite concentrations. |
OTHER | ||||||
| Methadone | 20 – 100 mg once a day | 300 mg once a day for 13 days | HIV infected patients (24) | ND | | |
| Ethinylestradiol (EE)/ Norethindrone (NE) | 35 mg EE / 1 mg NE for 21 days | 300 mg once a day for 10 days | Healthy female subjects (22) | ND | EE: ↓ AUC by 35%, ↓ Cmax by 20% NE: ↓ AUC by 46% | Patients should be advised to use additional or alternative methods of contraception. |
| Theophylline | 5 mg/kg | 300 mg for 14 days | Healthy subjects (11) | ND | | |
↑ indicates increase; ↓ indicates decrease; ↔ indicates no significant change
ND-No Data
AUC-Area under the Concentration vs. Time Curve;
Cmax-Maximum serum concentration; Cmin- Minimum serum concentration
a compared to rifabutin 300 mg once a day alone
b compared to historical control (fosamprenavir/ritonavir 700/100 mg twice a day)
c also taking zidovudine 500 mg once a day
d compared to rifabutin 150 mg once a day alone
e compared to rifabutin 300 mg once a day alone
f data from a case report
g compared to voriconazole 200 mg twice a day alone
The structurally similar drug, rifampin, is known to reduce the plasma concentrations of a number of other drugs (see prescribing information for rifampin). Although a weaker enzyme inducer than rifampin, rifabutin may be expected to have some effect on those drugs as well.
Rifabutin capsules for oral administration contain 150 mg of the rifamycin antimycobacterial agent rifabutin, USP, per capsule along with the inactive ingredients, microcrystalline cellulose, sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate. The hard gelatin capsule contains titanium dioxide, red iron oxide, gelatin, sodium lauryl sulfate and purified water. The imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide and purified water.
The chemical name for rifabutin is 1',4-didehydro-1-deoxy-1,4-dihydro-5'-(2-methylpropyl)-1-oxorifamycin XIV (Chemical Abstracts Service, 9th Collective Index) or (9
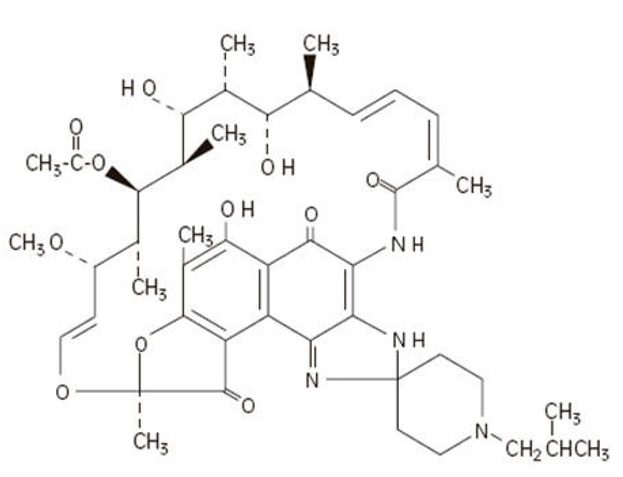
Rifabutin is a red-violet powder soluble in methanol, slightly soluble in ethanol, and slightly soluble in water (0.21 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water).