Ritonavir Prescribing Information
• When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including contraindication information.
• Ritonavir is contraindicated in patients with known hypersensitivity (e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome) to ritonavir or any of its ingredients.
• Ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions
o Alpha 1- Adrenoreceptor Antagonist : alfuzosin
o Antianginal: ranolazine
o Antiarrhythmics: amiodarone, dronedarone, flecainide, propafenone, quinidine
o Antifungal: voriconazole
o Anti-gout: colchicine
o Antipsychotics: lurasidone, pimozide
o Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
o GI Motility Agent: cisapride
o HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
o Microsomal triglyceride transfer protein (MTTP) Inhibitor: lomitapide
o PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary arterial hypertension
o Sedative/Hypnotics: triazolam, orally administered midazolam
• Ritonavir is contraindicated with drugs that are potent CYP3A inducers where significantly reduced ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross-resistance
o Anticancer Agents: apalutamide
o Herbal Products: St. John's Wort (hypericum perforatum)
• Ritonavir is contraindicated in patients with known hypersensitivity to ritonavir (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome) or any of its ingredients
• Co-administration with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations may be associated with serious and/or life-threatening events
• Co-administration with drugs that significantly reduce ritonavir
Initiation of ritonavir, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or initiation of medications metabolized by CYP3A in patients already receiving ritonavir, may increase plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or induce CYP3A may increase or decrease concentrations of ritonavir, respectively. These interactions may lead to:
• Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal events from greater exposures of concomitant medications.
• Clinically significant adverse reactions from greater exposures of ritonavir.
• Loss of therapeutic effect of ritonavir and possible development of resistance.
When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including important Warnings and Precautions.
See Table 4 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations
Ritonavir tablets are indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
• Adult patients: 600 mg twice-day with meals
The recommended dosage of ritonavir is 600 mg twice daily by mouth to be taken with meals. Use of a dose titration schedule may help to reduce treatment-emergent adverse events while maintaining appropriate ritonavir plasma levels. Ritonavir should be started at no less than 300 mg twice daily and increased at 2 to 3 day intervals by 100 mg twice daily. The maximum dose of 600 mg twice daily should not be exceeded upon completion of the titration
Ritonavir oral solution is not recommended during pregnancy due to its alcohol content. Ritonavir oral solution contains the excipients alcohol and propylene glycol
Ritonavir oral solution should not be administered to neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 44 weeks has been attained
Ritonavir oral solution contains the excipients alcohol and propylene glycol. Special attention should be given to accurate calculation of the dose of ritonavir, transcription of the medication order, dispensing information and dosing instructions to minimize the risk for medication errors, and overdose. This is especially important for young children. Total amounts of alcohol and propylene glycol from all medicines that are to be given to pediatric patients 1 to 6 months of age should be taken into account in order to avoid toxicity from these excipients
Body Surface Area (m2) | Twice Daily Dose 250 mg per m2 | Twice Daily Dose 300 mg per m2 | Twice Daily Dose 350 mg per m2 | Twice Daily Dose 400 mg per m2 |
| 0.20 | 0.6 mL (50 mg) | 0.75 mL (60 mg) | 0.9 mL (70 mg) | 1 mL (80 mg) |
| 0.25 | 0.8 mL (62.5 mg) | 0.9 mL (75 mg) | 1.1 mL (87.5 mg) | 1.25 mL (100 mg) |
| 0.50 | 1.6 mL (125 mg) | 1.9 mL (150 mg) | 2.2 mL (175 mg) | 2.5 mL (200 mg) |
| 0.75 | 2.3 mL (187.5 mg) | 2.8 mL (225 mg) | 3.3 mL (262.5 mg) | 3.75 mL (300 mg) |
| 1 | 3.1 mL (250 mg) | 3.75 mL (300 mg) | 4.4 mL (350 mg) | 5 mL (400 mg) |
| 1.25 | 3.9 mL (312.5 mg) | 4.7 mL (375 mg) | 5.5 mL (437.5 mg) | 6.25 mL (500 mg) |
| 1.50 | 4.7 mL (375 mg) | 5.6 mL (450 mg) | 6.6 mL (525 mg) | 7.5 mL (600 mg) |
| *The concentration of the oral solution is 80 mg per mL. | ||||
Body surface area (BSA) can be calculated as follows1: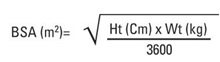

• Ritonavir oral solution should not be administered to neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 44 weeks has been attained (
Ritonavir must be used in combination with other antiretroviral agents
Ritonavir oral solution should not be administered to neonates before a postmenstrual age (first day of the mother’s last menstrual period to birth plus the time elapsed after birth) of 44 weeks has been attained
Ritonavir oral solution contains the excipients alcohol and propylene glycol. Special attention should be given to accurate calculation of the dose of ritonavir, transcription of the medication order, dispensing information and dosing instructions to minimize the risk for medication errors, and overdose. This is especially important for young children. Total amounts of alcohol and propylene glycol from all medicines that are to be given to pediatric patients 1 to 6 months of age should be taken into account in order to avoid toxicity from these excipients
Body Surface Area (m2) | Twice Daily Dose 250 mg per m2 | Twice Daily Dose 300 mg per m2 | Twice Daily Dose 350 mg per m2 | Twice Daily Dose 400 mg per m2 |
| 0.20 | 0.6 mL (50 mg) | 0.75 mL (60 mg) | 0.9 mL (70 mg) | 1 mL (80 mg) |
| 0.25 | 0.8 mL (62.5 mg) | 0.9 mL (75 mg) | 1.1 mL (87.5 mg) | 1.25 mL (100 mg) |
| 0.50 | 1.6 mL (125 mg) | 1.9 mL (150 mg) | 2.2 mL (175 mg) | 2.5 mL (200 mg) |
| 0.75 | 2.3 mL (187.5 mg) | 2.8 mL (225 mg) | 3.3 mL (262.5 mg) | 3.75 mL (300 mg) |
| 1 | 3.1 mL (250 mg) | 3.75 mL (300 mg) | 4.4 mL (350 mg) | 5 mL (400 mg) |
| 1.25 | 3.9 mL (312.5 mg) | 4.7 mL (375 mg) | 5.5 mL (437.5 mg) | 6.25 mL (500 mg) |
| 1.50 | 4.7 mL (375 mg) | 5.6 mL (450 mg) | 6.6 mL (525 mg) | 7.5 mL (600 mg) |
| *The concentration of the oral solution is 80 mg per mL. | ||||
Body surface area (BSA) can be calculated as follows1:

Ritonavir oral solution contains the excipients alcohol and propylene glycol. When administered concomitantly with propylene glycol, alcohol competitively inhibits the metabolism of propylene glycol, which may lead to elevated concentrations. Preterm neonates may be at an increased risk of propylene glycol-associated adverse events due to diminished ability to metabolize propylene glycol, thereby leading to accumulation and potential adverse events. Postmarketing life-threatening cases of cardiac toxicity (including complete AV block, bradycardia, and cardiomyopathy), lactic acidosis, acute renal failure, CNS depression and respiratory complications leading to death have been reported, predominantly in preterm neonates receiving lopinavir/ritonavir oral solution which also contains the excipients ethanol and propylene glycol.
Ritonavir oral solution should not be used in preterm neonates in the immediate postnatal period because of possible toxicities. However, if the benefit of using ritonavir oral solution to treat HIV infection in infants immediately after birth outweighs the potential risks, infants should be monitored closely for increases in serum osmolality and serum creatinine, and for toxicity related to ritonavir oral solution including: hyperosmolality, with or without lactic acidosis, renal toxicity, CNS depression (including stupor, coma, and apnea), seizures, hypotonia, cardiac arrhythmias and ECG changes, and hemolysis. Total amounts of ethanol and propylene glycol from all medicines that are to be given to infants should be taken into account in order to avoid toxicity from these excipients
• Dose modification for ritonavir is necessary when used with other protease inhibitors (
Dose reduction of ritonavir is necessary when used with other protease inhibitors: atazanavir, darunavir, fosamprenavir, saquinavir, and tipranavir.
Prescribers should consult the full prescribing information and clinical study information of these protease inhibitors if they are co-administered with a reduced dose of ritonavir
• Ritonavir Tablets USP, 100 mg
White to off white, capsule shaped, film coated tablets debossed with 'H' on one side and 'R9' on other side.
When co-administering ritonavir with other protease inhibitors, see the full prescribing information for the co-administered protease inhibitor including important information for use in special populations.
• When co-administering ritonavir with other protease inhibitors, see the full prescribing information for that protease inhibitor including contraindication information.
• Ritonavir is contraindicated in patients with known hypersensitivity (e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome) to ritonavir or any of its ingredients.
• Ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening reactions
Ritonavir is an inhibitor of cytochrome P450 3A (CYP3A) and may increase plasma concentrations of agents that are primarily metabolized by CYP3A. Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (greater than 3-fold) when co-administered with ritonavir. Thus, co-administration of ritonavir with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 4.
Ritonavir also inhibits CYP2D6 to a lesser extent. Co-administration of substrates of CYP2D6 with ritonavir could result in increases (up to 2-fold) in the AUC of the other agent, possibly requiring a proportional dosage reduction. Ritonavir also appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6 as well as other enzymes, including glucuronosyl transferase.
These examples are a guide and not considered a comprehensive list of all possible drugs that may interact with ritonavir. The healthcare provider should consult appropriate references for comprehensive information.
The pharmacokinetics of ritonavir have been studied in healthy volunteers and HIV-infected patients (CD4greater than or equal to 50 cells per µL). See Table 5 for ritonavir pharmacokinetic characteristics.
The absolute bioavailability of ritonavir has not been determined. After a 600 mg dose of oral solution, peak concentrations of ritonavir were achieved approximately 2 hours and 4 hours after dosing under fasting and non-fasting (514 KCal; 9% fat, 12% protein, and 79% carbohydrate) conditions, respectively.
Ritonavir tablets are not bioequivalent to ritonavir capsules. Under moderate fat conditions (857 kcal; 31% fat, 13% protein, 56% carbohydrates), when a single 100 mg ritonavir dose was administered as a tablet compared with a capsule, AUC(0 to ∞) met equivalence criteria but mean Cmaxwas increased by 26% (92.8% confidence intervals: ↑15 to ↑39%).
No information is available comparing ritonavir tablets to ritonavir capsules under fasting conditions.
The bioavailability of ritonavir tablet and oral solution is decreased under fed conditions as compared to fasted conditions.
Following the administration of a 100 mg tablet dose of ritonavir, Cmaxand AUCinfof ritonavir were decreased by 21 to 23% under moderate fat (857 Kcal, 30% from fat) or high fat conditions (917 Kcal, 60% calories from fat) relative to fasting conditions.
Following the administration of a 600 mg dose ritonavir oral solution, Cmaxand AUCinfof ritonavir were decreased by 23% and 7%, respectively, under nonfasting conditions (514 Kcal, 10% from fat) relative to fasting conditions. Dilution of the oral solution, within one hour of administration, with 240 mL of chocolate milk, Advera® or Ensure® did not significantly affect the extent and rate of ritonavir absorption.
Nearly all of the plasma radioactivity after a single oral 600 mg dose of 14C-ritonavir oral solution (n = 5) was attributed to unchanged ritonavir. Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite and has antiviral activity similar to that of parent drug; however, the concentrations of this metabolite in plasma are low.
In a study of five subjects receiving a 600 mg dose of 14C-ritonavir oral solution, 11.3 ± 2.8% of the dose was excreted into the urine, with 3.5 ± 1.8% of the dose excreted as unchanged parent drug. In that study, 86.4 ± 2.9% of the dose was excreted in the feces with 33.8 ± 10.8% of the dose excreted as unchanged parent drug. Upon multiple dosing, ritonavir accumulation is less than predicted from a single dose possibly due to a time and dose-related increase in clearance.
Parameter | N | Values (Mean ± SD) |
| Vβ/F‡ | 91 | 0.41 ± 0.25 L/kg |
| t½ | 3 - 5 h | |
| CL/F SS† | 10 | 8.8 ± 3.2 L/h |
| CL/F‡ | 91 | 4.6 ± 1.6 L/h |
| CLR | 62 | < 0.1 L/h |
| RBC/Plasma Ratio | 0.14 | |
| Percent Bound* | 98 to 99% | |
| † SS = steady state; patients taking ritonavir 600 mg q12h. ‡ Single ritonavir 600 mg dose. * Primarily bound to human serum albumin and alpha-1 acid glycoprotein over the ritonavir concentration range of 0.01 to 30 mcg/mL. | ||
No age-related pharmacokinetic differences have been observed in adult patients (18 to 63 years). Ritonavir pharmacokinetics have not been studied in older patients.
A study of ritonavir pharmacokinetics in healthy males and females showed no statistically significant differences in the pharmacokinetics of ritonavir. Pharmacokinetic differences due to race have not been identified.
Steady-state pharmacokinetics were evaluated in 37 HIV-infected patients ages 2 to 14 years receiving doses ranging from 250 mg per m2twice-daily to 400 mg per m2twice-daily in PACTG Study 310, and in 41 HIV-infected patients ages 1 month to 2 years at doses of 350 and 450 mg per m2twice-daily in PACTG Study 345. Across dose groups, ritonavir steady-state oral clearance (CL/F/m2) was approximately 1.5 to 1.7 times faster in pediatric patients than in adult subjects. Ritonavir concentrations obtained after 350 to 400 mg per m2twice-daily in pediatric patients greater than 2 years were comparable to those obtained in adults receiving 600 mg (approximately 330 mg per m2) twice-daily. The following observations were seen regarding ritonavir concentrations after administration with 350 or 450 mg per m2twice-daily in children less than 2 years of age. Higher ritonavir exposures were not evident with 450 mg per m2 twice-daily compared to the 350 mg per m2 twice-daily. Ritonavir trough concentrations were somewhat lower than those obtained in adults receiving 600 mg twice-daily. The area under the ritonavir plasma concentration time curve and trough concentrations obtained after administration with 350 or 450 mg per m2twice-daily in children less than 2 years were approximately 16% and 60% lower, respectively, than that obtained in adults receiving 600 mg twice daily.
Ritonavir pharmacokinetics have not been studied in patients with renal impairment, however, since renal clearance is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Dose-normalized steady-state ritonavir concentrations in subjects with mild hepatic impairment (400 mg twice-daily, n = 6) were similar to those in control subjects dosed with 500 mg twice-daily. Dose-normalized steady-state ritonavir exposures in subjects with moderate hepatic impairment (400 mg twice-daily, n= 6) were about 40% lower than those in subjects with normal hepatic function (500 mg twice-daily, n = 6). Protein binding of ritonavir was not statistically significantly affected by mild or moderately impaired hepatic function. No dose adjustment is recommended in patients with mild or moderate hepatic impairment. However, health care providers should be aware of the potential for lower ritonavir concentrations in patients with moderate hepatic impairment and should monitor patient response carefully. Ritonavir has not been studied in patients with severe hepatic impairment.
Based on evaluation of the published literature, ritonavir exposures are reduced during pregnancy relative to postpartum.
Table 6 and Table 7 summarize the effects on AUC and Cmax, with 95% confidence intervals (95% CI), of co-administration of ritonavir with a variety of drugs. For information about clinical recommendations see Table 4 in
Co-administered Drug | Dose of Co- administered Drug (mg) | Dose of Ritonavir (mg) | N | AUC % (95% CI) | Cmax (95% CI) | Cmin (95% CI) |
| Clarithromycin | 500 q12h, 4 d | 200 q8h, 4 d | 22 | ↑ 12% (2, 23%) | ↑ 15% (2, 28%) | ↑ 14% (-3, 36%) |
| Didanosine | 200 q12h, 4 d | 600 q12h, 4 d | 12 | ↔ | ↔ | ↔ |
| Fluconazole | 400 single dose, day 1; 200 daily, 4 d | 200 q6h, 4 d | 8 | ↑ 12% (5, 20%) | ↑ 15% (7, 22%) | ↑ 14% (0, 26%) |
| Fluoxetine | 30 q12h, 8 d | 600 single dose, 1 d | 16 | ↑ 19% (7, 34%) | ↔ | ND |
| Ketoconazole | 200 daily, 7 d | 500 q12h, 10 d | 12 | ↑ 18% (-3, 52%) | ↑ 10% (-11, 36%) | ND |
| Rifampin | 600 or 300 daily, 10 d | 500 q12h, 20 d | 7, 9* | ↓ 35% (7, 55%) | ↓ 25% (-5, 46%) | ↓ 49% (-14, 91%) |
| Voriconazole | 400 q12h, 1 d; then 200 q12h, 8 d | 400 q12h, 9 d | ↔ | ↔ | ND | |
| Zidovudine | 200 q8h, 4 d | 300 q6h, 4 d | 10 | ↔ | ↔ | ↔ |
| ND=not determined | ||||||
Co-administered Drug | Dose of Co- administered Drug (mg) | Dose of Ritonavir (mg) | N | AUC % (95% CI) | Cmax (95% CI) | Cmin (95% CI) |
Alprazolam | 1, single dose | 500 q12h,10 d | 12 | ↓ 12% (-5, 30%) | ↓ 16 % (5, 27%) | ND |
Avanafil | 50, single dose | 600 q12h | 146 | ↑ 13-fold | ↑ 2.4-fold | ND |
| Clarithromycin 14-OH clarithromycin metabolite | 500 q12h, 4 d | 200 q8h, 4 d | 22 | ↑ 77% (56, 103%) ↓ 100% | ↑ 31% (15, 51%) ↓ 99% | ↑ 2.8-fold (2.4, 3.3X) ↓ 100% |
| Desipramine 2-OH desipramine metabolite | 100, single dose | 500 q12h, 12 d | 14 | ↑ 145% (103, 211%) ↓ 15% (3, 26 %) | ↑ 22% (12, 35%) ↓ 67% (62, 72%) | ND ND |
| Didanosine | 200 q12h, 4 d | 600 q12h, 4 d | 12 | ↓ 13% (0, 23%) | ↓ 16% (5, 26%) | ↔ |
| Ethinyl estradiol | 50 mcg single dose | 500 q12h, 16 d | 23 | ↓ 40% (31, 49%) | ↓ 32% (24, 39%) | ND |
| Fluticasone propionate aqueous nasal spray | 200 mcg qd, 7 d | 100 mg q12h, 7 d | 18 | ↑ approximately 350-fold5 | ↑ approximately 25-fold5 | |
| Indinavir1 Day 14 Day 15 | 400 q12h, 15 d | 400 q12h, 15 d | 10 | ↑ 6% (-14, 29%) ↓ 7% (-22, 28%) | ↓ 51% (40, 61%) ↓ 62% (52, 70%) | ↑ 4-fold (2.8, 6.8X) ↑ 4-fold (2.5, 6.5X) |
| Ketoconazole | 200 daily, 7 d | 500 q12h, 10 d | 12 | ↑ 3.4-fold (2.8, 4.3X) | ↑ 55% (40, 72%) | ND |
| Meperidine Normeperidine metabolite | 50 oral single dose | 500 q12h, 10 d | 8 6 | ↓ 62% (59, 65%) ↑ 47% (-24, 345%) | ↓ 59% (42, 72%) ↑ 87% (42, 147%) | ND ND |
| Methadone2 | 5, single dose | 500 q 12h, 15 d | 11 | ↓ 36% (16, 52%) | ↓ 38% (28, 46%) | ND |
| Raltegravir | 400, single dose | 100 q12h, 16 d | 10 | ↓ 16% (-30, 1%) | ↓ 24% (-45, 4%) | ↓ 1% (-30, 40%) |
| Rivaroxaban | 10, single dose (days 0 and 7) | 600 q12h (days 2 to 7) | 12 | ↑ 150% (130-170%)7 | ↑ 60% (40-70%)7 | ND |
| Rifabutin 25- O -desacetylrifabutin metabolite | 150 daily, 16 d | 500 q12h, 10 d | 5, 11* | ↑ 4-fold (2.8, 6.1X) ↑ 38-fold (28, 56X) | ↑ 2.5-fold (1.9, 3.4X) ↑ 16-fold (13, 20X) | ↑ 6-fold (3.5, 18.3X) ↑ 181- fold (ND) |
| Sildenafil | 100, single dose | 500 twice daily, 8 d | 28 | ↑ 11-fold | ↑ 4-fold | ND |
| Simeprevir | 200 mg qd, 7 d | 100 mg bid, 15 d | 12 | ↑ 618% (463%-815%)8 | ↑370% (284%- 476%)8 | ↑1335% (929%-1901%)8 |
| Sulfamethoxazole3 | 800, single dose | 500 q12h, 12 d | 15 | ↓ 20% (16, 23%) | ↔ | ND |
| Tadalafil | 20 mg, single dose | 200 mg q12h | ↑ 124% | ↔ | ND | |
| Theophylline | 3 mg/kg q8h, 15 d | 500 q12h, 10 d | 13, 11* | ↓ 43% (42, 45%) | ↓ 32% (29, 34%) | ↓57% (55, 59%) |
| Trazodone | 50 mg, single dose | 200 mg q12h, 4 doses | 10 | ↑ 2.4-fold | ↑ 34% | |
| Trimethoprim3 | 160, single dose | 500 q12h, 12 d | 15 | ↑ 20% (3, 43%) | ↔ | ND |
| Vardenafil | 5 mg | 600 q12h | ↑ 49-fold | ↑ 13-fold | ND | |
| Voriconazole | 400 q12h, 1 d; then 200 q12h, 8 d | 400 q12h, 9 d | ↓ 82% | ↓ 66% | ||
| 400 q12h, 1 d; then 200 q12h, 8 d | 100 q12h, 9 d | ↓ 39% | ↓ 24% | |||
| Warfarin S-Warfarin R-Warfarin | 5, single dose | 400 q12h, 12d | 12 | ↑ 9% (-17, 44%)4 ↓ 33% (-38, -27%)4 | ↓ 9% (-16, -2%)4 ↔ | ND ND |
| Zidovudine | 200 q8h, 4 d | 300 q6h, 4 d | 9 | ↓ 25% (15, 34%) | ↓ 27% (4, 45%) | ND |
ND=not determined 1 Ritonavir and indinavir were co-administered for 15 days; Day 14 doses were administered after a 15%-fat breakfast (757 Kcal) and 9%-fat evening snack (236 Kcal), and Day 15 doses were administered after a 15%-fat breakfast (757 Kcal) and 32%-fat dinner (815 Kcal). Indinavir Cmin was also increased 4-fold. Effects were assessed relative to an indinavir 800 mg q8h regimen under fasting conditions. 2 Effects were assessed on a dose-normalized comparison to a methadone 20 mg single dose. 3 Sulfamethoxazole and trimethoprim taken as single combination tablet. 4 90% CI presented for R- and S-warfarin AUC and Cmax ratios. 5 This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in plasma cortisol AUC. 6 For the reference arm: N=14 for Cmax and AUC (0 to inf), and for the test arm: N=13 for Cmax and N=4 for AUC (0 to inf). 7 90% CI presented for rivaroxaban 8 90% CI presented for simeprevir (change in exposure presented as percentage increase) ↑ Indicates increase, ↓ indicates decrease, ↔ indicates no change. * Parallel group design; entries are subjects receiving combination and control regimens, respectively. | ||||||
o Alpha 1- Adrenoreceptor Antagonist : alfuzosin
o Antianginal: ranolazine
o Antiarrhythmics: amiodarone, dronedarone, flecainide, propafenone, quinidine
o Antifungal: voriconazole
o Anti-gout: colchicine
o Antipsychotics: lurasidone, pimozide
o Ergot Derivatives: dihydroergotamine, ergotamine, methylergonovine
o GI Motility Agent: cisapride
o HMG-CoA Reductase Inhibitors: lovastatin, simvastatin
o Microsomal triglyceride transfer protein (MTTP) Inhibitor: lomitapide
o PDE5 Inhibitor: sildenafil (Revatio®) when used for the treatment of pulmonary arterial hypertension
o Sedative/Hypnotics: triazolam, orally administered midazolam
• Ritonavir is contraindicated with drugs that are potent CYP3A inducers where significantly reduced ritonavir plasma concentrations may be associated with the potential for loss of virologic response and possible resistance and cross-resistance
o Anticancer Agents: apalutamide
o Herbal Products: St. John's Wort (hypericum perforatum)