Rivfloza
(Nedosiran)Rivfloza Prescribing Information
RIVFLOZA is indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, e.g., eGFR ≥30 mL/min/1.73 m2
The pharmacokinetic (PK) properties of RIVFLOZA were evaluated following administration of single and multiple dosages in patients with PH1 or PH2 as summarized in
Nedosiran | ||
General Information | ||
Steady State Exposure | Cmax[Mean (%CV)] | 844 (44) ng/mL |
AUC0-last[Mean (%CV)] |
| |
Dose Proportionality | Nedosiran exhibited a dose-proportional increase in plasma exposure following single subcutaneous doses from 1.5 to 6.0 mg/kg. Nedosiran exhibited time-independent pharmacokinetics with multiple doses of 160 mg once monthly (body weight ≥50 kg), 128 mg once monthly (body weight <50 kg), or 3.3 mg/kg once monthly in the age range of 6 to 11 years. | |
Accumulation | No accumulation of nedosiran was observed in plasma following repeated monthly dosing. | |
Absorption | ||
Tmax[Median (Range)] | 6 (2 to 12) hours | |
Distributiona | ||
Estimated Vz/F | 126 L | |
Protein Binding | 85.6% | |
Elimination | ||
Half-Life (Mean (%CV)]) | 15 (68) hours | |
Estimated CL/F | 5.7 L/hr | |
Metabolism | ||
Primary Pathway | Nedosiran is metabolized by endo- and exonucleases to shorter oligonucleotides. | |
Excretion | ||
Primary Pathway | Approximately 27% of the administered nedosiran dose is excreted unchanged into the urine within 24 hours of dosing. | |
aNedosiran distributes primarily to the liver after subcutaneous administration. Cmax= maximum plasma concentration; AUC0-last= area under the plasma concentration-time curve from time of administration (0) to the last measurable time point (last); Tmax= time to maximum concentration; Vz/F = apparent volume of distribution; CV = coefficient of variation; CL/F = apparent clearance. | ||
No clinically significant differences in the pharmacokinetics or pharmacodynamics of nedosiran were observed based on age (2 to 73 years old), sex, race/ethnicity, mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m2)
At the recommended clinical dose, PK exposure of nedosiran is similar in adult and pediatric patients 2 years of age and older.
Concomitant use of pyridoxine (vitamin B6) did not have a significant impact on the PK of nedosiran.
In vitro studies demonstrated that nedosiran was not an inhibitor or inducer of cytochrome P450 (CYP) enzymes and was neither a substrate nor an inhibitor of efflux and uptake transporters.
PHYOX2 was a randomized, double-blind trial comparing RIVFLOZA and placebo in patients aged 6 years or older with PH1 or PH2 and an eGFR ≥ 30 mL/min/1.73 m2(NCT03847909). Too few PH2 patients were enrolled to evaluate efficacy in the PH2 population. Therefore, RIVFLOZA is only indicated for patients with PH1
Patients received monthly doses of RIVFLOZA (N=23) or placebo (N=12). The RIVFLOZA dose for patients at least 12 years of age weighing at least 50 kg was 160 mg, for patients at least 12 years of age weighing less than 50 kg was 128 mg, and for children 6 to 11 years of age was 3.3 mg/kg (to a maximum of 128 mg).
The median age was 20 years (range 9 - 46 years), 51% were female, 71% were White, 17% were Asian, 83% had PH1, and 17% had PH2. At baseline, mean 24-hour urinary oxalate excretion, normalized by 1.73 m2BSA in patients less than 18 years of age, was 1547 µmol/24‑hour. Mean plasma oxalate was 8.2 µmol/L, 43% of patients had an eGFR ≥ 90 mL/min/1.73 m2, 34% had an eGFR 60 to < 90 mL/min/1.73 m2, 23% had an eGFR 30 to < 60 mL/min/1.73 m2, and 60% were taking pyridoxine.
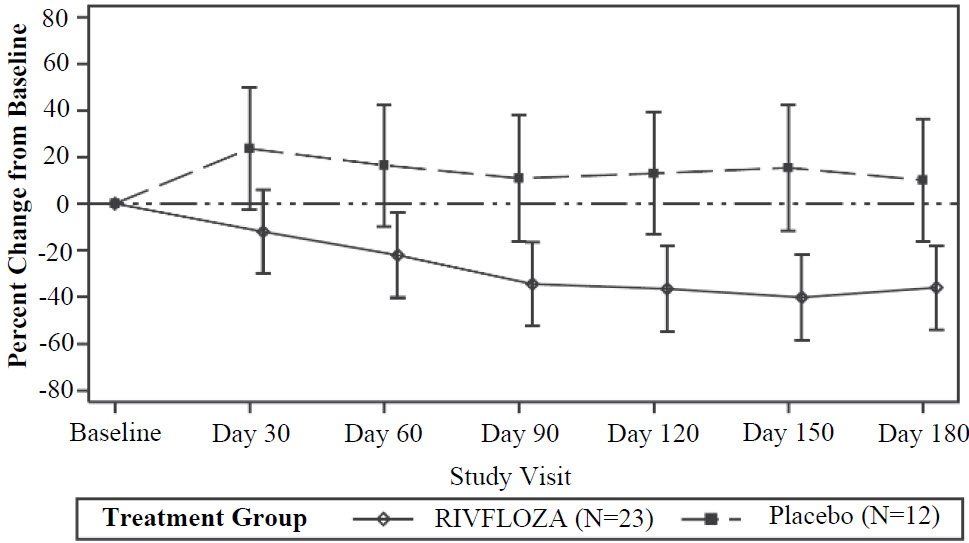
The primary efficacy endpoint was the area under the curve, from Days 90 to 180, of the percent change from baseline in 24-hour urinary oxalate excretion (AUC24-hour Uox). The least-squares (LS) mean AUC24‑hour Uoxwas -3486 (95% CI: -5025, -1947) in the RIVFLOZA group compared to 1490 (95% CI: 781, 3761) in the placebo group, for a between group difference of 4976 (95% CI: 2803, 7149; p<0.0001).
The LS mean percent change from baseline in 24-hour urinary oxalate excretion (corrected for BSA in patients < 18 years of age) averaged over Days 90, 120, 150 and 180, was -37% (95% CI: -53%, -21%) in the RIVFLOZA group and 12% (95% CI: ‑12%, 36%) in the placebo group, for a between group difference of 49% (95% CI: 26%, 72%) [
After 6 months of treatment in PHYOX2, patients could enroll in an ongoing single-arm extension study, PHYOX3 (NCT04042402), in which all patients were treated with RIVFLOZA. The reduction in urinary oxalate was maintained in the 13 patients with PH1 who received an additional 6 months of treatment in PHYOX3.

The recommended dosage is shown below and is administered subcutaneously once monthly. (
RIVFLOZA is administered subcutaneously once monthly at the recommended doses shown in
Dosing is based on actual body weight.
Body weight | |||
Less than 39 kg | 39 kg to less than 50 kg | 50 kg and above | |
Age 2 to less than 12 years | 3.3 mg/kg | 128 mg | 160 mg |
Age 12 years and older | 128 mg | 160 mg | |
If a planned dose is missed, administer RIVFLOZA as soon as possible. If the planned dose is missed by more than 7 days, administer RIVFLOZA as soon as possible and resume monthly dosing from the most recently administered dose.
Body weight | |||
Less than 39 kg | 39 kg to less than 50 kg | 50 kg and above | |
Age 2 to less than 12 years | 3.3 mg/kg | 128 mg | 160 mg |
Age 12 years and older | 128 mg | 160 mg | |
See full Prescribing Information for important administration instructions. (
RIVFLOZA is administered subcutaneously once monthly at the recommended doses shown in
Dosing is based on actual body weight.
Body weight | |||
Less than 39 kg | 39 kg to less than 50 kg | 50 kg and above | |
Age 2 to less than 12 years | 3.3 mg/kg | 128 mg | 160 mg |
Age 12 years and older | 128 mg | 160 mg | |
If a planned dose is missed, administer RIVFLOZA as soon as possible. If the planned dose is missed by more than 7 days, administer RIVFLOZA as soon as possible and resume monthly dosing from the most recently administered dose.
RIVFLOZA Injection 160 mg/mL (present as 170 mg nedosiran sodium) is a clear, colorless-to-yellow solution available as follows:
• 80 mg/0.5 mL single-dose vial• 128 mg/0.8 mL single-dose Pre-filled Syringe• 160 mg/ mL single-dose Pre-filled Syringe
Available data from reports of pregnancy in clinical trials with RIVFLOZA are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
In animal reproduction studies, no adverse developmental effects were observed when nedosiran was administered to pregnant mice at doses up to approximately 58 times the maximum recommended human dose (MRHD) of 160 mg nedosiran (equivalent to 170 mg nedosiran sodium) per dose, based on body surface area (BSA) or upon administration of a mouse-specific (pharmacologically active) analog. Subcutaneous administration of nedosiran to pregnant rabbits during the period of organogenesis at doses approximating the MRHD resulted in increased fetal loss in the presence of maternal toxicity. Adverse developmental outcomes (fetal cardiovascular and skeletal malformations) were observed at a dose approximately 2 times the MRHD
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
In mice, subcutaneous administration of nedosiran at doses up to 2000 mg/kg/dose (approximately 58 times the MRHD based on BSA) or a mouse-specific (pharmacologically active) analog (10 mg/kg/dose) during organogenesis (dosing on gestation days 6, 8, 10, 12, and 14 for nedosiran; gestation days 3 and 10 for the analog) did not have adverse effects on embryo-fetal development.
Subcutaneous administration of nedosiran (0, 2, 6 or 20 mg/kg/dose) to pregnant rabbits during organogenesis (dosing on gestation days 7, 9, 11, 13, 15, 17, and 19) resulted in maternal toxicity on the basis of body weight loss of up to 6.5% following the first dose in the 6 and 20 mg/kg/dose groups. Higher post-implantation loss and lower numbers of live fetuses occurred at ≥6 mg/kg/dose (exposures equivalent to the MRHD based on BSA), and fetal cardiovascular and skeletal malformations occurred at the 20 mg/kg/dose (2 times the MRHD based on BSA). At the 2 mg/kg/dose, which is below the MRHD, no adverse findings were seen.
In a pre- and postnatal study in mice, subcutaneous administration of nedosiran (0, 250, 500, or 1000 mg/kg/dose) or a mouse-specific (pharmacologically active) analog (10 mg/kg/dose) from implantation (dosing on gestational days 6, 8, 10, 12, 14, 16) to weaning (dosing on lactation days 1, 8, 15, 20) did not have adverse effects on the growth, viability, development and reproductive performance of the offspring
None.
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions. (
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RIVFLOZA has been evaluated in one placebo-controlled clinical trial (PHYOX2) and one open-label extension study (PHYOX3). Across these studies, 29 adults and 12 children with PH1 have been treated with RIVFLOZA. Patients with PH1 in these studies ranged in age from 9 to 46 years at first dose. The median duration of exposure was approximately 15 months (range 1-29 months). Overall, 38 patients with PH1 were treated for at least 6 months, 24 patients for at least 12 months, and 16 patients for at least 18 months.
In the randomized, placebo-controlled, double-blind PHYOX2 trial in pediatric and adult patients 9 to 46 years of age, 18 patients with PH1 received RIVFLOZA and 11 patients received placebo. Of the 18 patients treated with RIVFLOZA, 17 patients received ≥5 months of active treatment. The most common adverse reactions were injection site reactions, which were reported in 7 patients with PH1 (39%) on RIVFLOZA as compared to no patients on placebo. Injection site reactions included erythema, pain, bruising, and rash and were generally mild and did not lead to discontinuation of treatment.
In the single-arm extension study (PHYOX3) that included 40 patients with PH1, additional injection site reactions included atrophy in 1 patient (3%).
The safety of RIVFLOZA has additionally been evaluated in one single-arm clinical study (PHYOX8) in 15 pediatric patients 2 to less than 12 years of age with PH1 and an eGFR