Rizatriptan Benzoate - Rizatriptan Benzoate tablet prescribing information
INDICATIONS AND USAGE
Rizatriptan benzoate tablets are indicated for the acute treatment of migraine with or without aura in adults and in pediatric patients 6 to 17 years old. Limitations of Use
- Rizatriptan benzoate tablets should only be used where a clear diagnosis of migraine has been established. If a patient has no response for the first migraine attack treated with rizatriptan benzoate tablets, the diagnosis of migraine should be reconsidered before rizatriptan benzoate tablets are administered to treat any subsequent attacks.
- Rizatriptan benzoate tablets are not indicated for use in the management of hemiplegic or basilar migraine [see Contraindications (4) ] .
- Rizatriptan benzoate tablets are not indicated for the prevention of migraine attacks.
- Safety and effectiveness of rizatriptan benzoate tablets have not been established for cluster headache.
DOSAGE AND ADMINISTRATION
- Adults: 5 or 10 mg single dose; separate repeat doses by at least two hours; maximum dose in a 24-hour period: 30 mg (2.1 )
- Pediatric patients 6 to 17 years: 5 mg single dose in patients less than 40 kg (88 lb); 10 mg single dose in patients 40 kg (88 lb) or more (2.2 )
- Adjust dose if co-administered with propranolol (2.4 )
Dosing Information in Adults
The recommended starting dose of rizatriptan benzoate tablets are either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10-mg dose may provide a greater effect than the 5-mg dose, but may have a greater risk of adverse reactions [see Clinical Studies (14.1) ] . Redosing in Adults Although the effectiveness of a second dose or subsequent doses has not been established in placebo-controlled trials, if the migraine headache returns, a second dose may be administered 2 hours after the first dose. The maximum daily dose should not exceed 30 mg in any 24-hour period. The safety of treating, on average, more than four headaches in a 30-day period has not been established.
Dosing Information in Pediatric Patients (Age 6 to 17 Years)
Dosing in pediatric patients is based on the patient's body weight. The recommended dose of rizatriptan benzoate tablets is 5 mg in patients weighing less than 40 kg (88 lb), and 10 mg in patients weighing 40 kg (88 lb) or more.
The efficacy and safety of treatment with more than one dose of rizatriptan benzoate tablet within 24 hours in pediatric patients 6 to 17 years of age have not been established.
Dosage Adjustment for Patients on Propranolol
Adult Patients In adult patients taking propranolol, only the 5-mg dose of rizatriptan benzoate tablets are recommended, up to a maximum of 3 doses in any 24-hour period (15 mg) [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ] .
Pediatric Patients
For pediatric patients weighing 40 kg (88 lb) or more, taking propranolol, only a single 5-mg dose of rizatriptan benzoate tablets is recommended (maximum dose of 5 mg in a 24-hour period). Rizatriptan benzoate tablets should not be prescribed to propranolol-treated pediatric patients who weigh less than 40 kg (88 lb) [see Drug Interactions (7.1 ) and Clinical Pharmacology (12.3 )].
DOSAGE FORMS AND STRENGTHS
Rizatriptan benzoate Tablets, USP
- 5 mg tablets are white to off-white, capsule-shaped, compressed tablets debossed “RZT” on one side and “5” on other side.
- 10 mg tablets are white to off-white, capsule-shaped, compressed tablets debossed “RZT” on one side and “10” on other side.
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm (8.1 )
Pregnancy
Risk Summary Available human data on the use of rizatriptan benzoate tablets in pregnant women are not sufficient to draw conclusions about drug-associated risk for major birth defects and miscarriage. In animal studies, developmental toxicity was observed following oral administration of rizatriptan during pregnancy (decreased fetal body weight in rats) or throughout pregnancy and lactation (increased mortality, decreased body weight, and neurobehavioral impairment in rat offspring) at maternal plasma exposures greater than that expected at therapeutic doses in humans [see Animal Data]. In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The reported rate of major birth defects among deliveries to women with migraine range from 2.2% to 2.9% and the reported rate of miscarriage was 17%, which are similar to rates reported in women without migraine. Clinical Considerations Disease-Associated Maternal and/or Embryo/Fetal Risk In women with migraine, there is an increased risk of adverse perinatal outcomes in the mother, including pre-eclampsia and gestational hypertension. Data Human Data The Pregnancy Registry for rizatriptan benzoate tablets did not identify any pattern of congenital anomalies or other adverse birth outcomes over the period of 1998 to 2018. However, the lack of identification of any pattern should be viewed with caution, as the number of prospective reports with outcome information was low and did not provide sufficient power to detect an increased risk of individual birth defects associated with the use of rizatriptan benzoate tablets. Additionally, there was significant loss to follow-up in the prospective pregnancy reports, further complicating this assessment of an association between rizatriptan benzoate tablets and any pattern of congenital anomalies or other adverse birth outcomes. In a study using data from the Swedish Medical Birth Register, live births to women who reported using triptans or ergots during pregnancy were compared with those of women who did not. Of the 157 births with first-trimester exposure to rizatriptan, 7 infants were born with malformations (relative risk 1.01 [95% CI: 0.40 to 2.08]). A study using linked data from the Medical Birth Registry of Norway to the Norwegian Prescription Database compared pregnancy outcomes in women who redeemed prescriptions for triptans during pregnancy, as well as a migraine disease comparison group who redeemed prescriptions for triptans before pregnancy only, compared with a population control group. Of the 310 women who redeemed prescriptions for rizatriptan during the first trimester, 10 had infants with major congenital malformations (OR 1.03 [95% CI: 0.55 to 1.93]), while for the 271 women who redeemed prescriptions for rizatriptan before, but not during, pregnancy, 12 had infants with major congenital malformations (OR 1.48 [95% CI: 0.83 to 2.64]), each compared with the population comparison group. Animal Data When rizatriptan (0, 2, 10, or 100 mg/kg/day) was administered orally to pregnant rats throughout organogenesis, a decrease in fetal body weight was observed at the highest doses tested. At the mid dose (10 mg/kg/day), which was a no-effect dose for adverse effects on embryofetal development, plasma exposure (AUC) was approximately 15 times that in humans at the maximum recommended human dose (MRHD) of 30 mg/day. When rizatriptan (0, 5, 10, or 50 mg/kg/day) was administered orally to pregnant rabbits throughout organogenesis, no adverse fetal effects were observed. Plasma exposure (AUC) at the highest dose tested was 115 times that in humans at the MRHD. Placental transfer of drug to the fetus was demonstrated in both species. Oral administration of rizatriptan (0, 2, 10, or 100 mg/kg/day) to female rats prior to and during mating and continuing throughout gestation and lactation resulted in reduced body weight in offspring from birth and throughout lactation at all but the lowest dose tested (2 mg/kg/day). Plasma exposure (AUC) at the no-effect dose (2 mg/kg/day) for adverse effects on postnatal development was similar to that in humans at the MRHD. Oral administration of rizatriptan (0, 5, 100, or 250 mg/kg/day) throughout organogenesis and lactation resulted in neonatal mortality, reduced body weight (which persisted into adulthood), and impaired neurobehavioral function in offspring at all but the lowest dose tested. Plasma exposure (AUC) at the no-effect dose for adverse effects on postnatal development (5 mg/kg/day) was approximately 8 times that in humans at the MRHD.
Lactation
Risk Summary There are no data on the presence of rizatriptan or any active metabolites in human milk, or on the effects of rizatriptan on the breastfed infant, or on milk production. Rizatriptan was excreted in rat milk, with levels in milk approximately 6 times those in maternal plasma. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rizatriptan benzoate and any potential adverse effects on the breastfed infant from rizatriptan benzoate from the underlying maternal condition. Data Following oral administration of rizatriptan to lactating rats at a dose of 100 mg/kg/day, drug concentrations of rizatriptan in milk samples exceeded maternal plasma drug concentrations by approximately 6-fold.
Pediatric Use
Safety and effectiveness in pediatric patients under 6 years of age have not been established.
The efficacy and safety of rizatriptan benzoate tablets in the acute treatment of migraine in patients aged 6 to 17 years was established in an adequate and well-controlled study [see Clinical Studies (14.2) ] .
The incidence of adverse reactions reported for pediatric patients in the acute clinical trial was similar in patients who received rizatriptan benzoate tablets to those who received placebo. The adverse reaction pattern in pediatric patients is expected to be similar to that in adults.
Geriatric Use
Clinical studies of rizatriptan benzoate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Although the pharmacokinetics of rizatriptan were similar in elderly (aged ≥65 years) and in younger adults (n=17), in general, dose selection for an elderly patient should be cautious, starting at the low end of the dosing range. This reflects the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of coronary artery disease) should have a cardiovascular evaluation prior to receiving rizatriptan benzoate tablets [see Warnings and Precautions (5.1) ] .
CONTRAINDICATIONS
Rizatriptan benzoate tablets are contraindicated in patients with:
- Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), or other significant underlying cardiovascular disease [see Warnings and Precautions (5.1) ] .
- Coronary artery vasospasm including Prinzmetal's angina [see Warnings and Precautions(5.1) ] .
- History of stroke or transient ischemic attack (TIA) [see Warnings and Precautions (5.4) ] .
- Peripheral vascular disease (PVD) [see Warnings and Precautions (5.5) ] .
- Ischemic bowel disease [see Warnings and Precautions (5.5) ] .
- Uncontrolled hypertension [see Warnings and Precautions (5.8) ] .
- Recent use (i.e., within 24 hours) of another 5-HT 1 agonist, ergotamine-containing medication, or ergot-type medication (such as dihydroergotamine or methysergide) [see Drug Interactions (7.2 and 7.3) ] .
- Hemiplegic or basilar migraine [see Indications and Usage (1 )].
- Concurrent administration or recent discontinuation (i.e., within 2 weeks) of a MAO-A inhibitor [see Drug Interactions (7.5) and Clinical Pharmacology (12.3) ] .
- Hypersensitivity to rizatriptan or any of the excipients (angioedema and anaphylaxis seen) [see Adverse Reactions (6.2) ] .
WARNINGS AND PRECAUTIONS
- Myocardial ischemia, myocardial infarction, and Prinzmetal's angina: Perform cardiac evaluation in patients with multiple cardiovascular risk factors (5.1 )
- Arrhythmias: Discontinue dosing if occurs (5.2 )
- Chest/throat/neck/jaw pain, tightness, pressure, or heaviness; Generally not associated with myocardial ischemia; Evaluate patients at high risk (5.3 )
- Cerebral hemorrhage, subarachnoid hemorrhage, and stroke: Discontinue dosing if occurs (5.4 )
- Gastrointestinal ischemic events, peripheral vasospastic reactions: Discontinue dosing if occurs (5.5 )
- Medication overuse headache: Detoxification may be necessary (5.6 )
- Serotonin syndrome: Discontinue dosing if occurs (5.7 )
Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina
Rizatriptan benzoate tablets should not be given to patients with ischemic or vasospastic coronary artery disease. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of rizatriptan benzoate tablets. Some of these reactions occurred in patients without known coronary artery disease (CAD). 5-HT 1 agonists, including rizatriptan benzoate tablets may cause coronary artery vasospasm (Prinzmetal's Angina), even in patients without a history of CAD. Triptan-naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) should have a cardiovascular evaluation prior to receiving rizatriptan benzoate tablets. If there is evidence of CAD or coronary artery vasospasm, rizatriptan benzoate tablets should not be administered [see Contraindications (4) ] . For patients who have a negative cardiovascular evaluation, consideration should be given to administration of the first rizatriptan benzoate tablets dose in a medically supervised setting and performing an electrocardiogram (ECG) immediately following rizatriptan benzoate tablets administration. Periodic cardiovascular evaluation should be considered in intermittent long-term users of rizatriptan benzoate tablets who have cardiovascular risk factors.
Arrhythmias
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT 1 agonists. Discontinue rizatriptan benzoate tablets if these disturbances occur.
Chest, Throat, Neck and/or Jaw Pain/Tightness/Pressure
As with other 5-HT 1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck and jaw commonly occur after treatment with rizatriptan benzoate tablets and are usually non-cardiac in origin. However, if a cardiac origin is suspected, patients should be evaluated. Patients shown to have CAD and those with Prinzmetal's variant angina should not receive 5-HT 1 agonists.
Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT 1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT 1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). Discontinue rizatriptan benzoate tablets, USP if a cerebrovascular event occurs. As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, care should be taken to exclude other potentially serious neurological conditions. Rizatriptan benzoate tablets should not be administered to patients with a history of stroke or transient ischemic attack [see Contraindications (4) ] .
Other Vasospasm Reactions
5-HT 1 agonists, including rizatriptan benzoate tablets, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT 1 agonist, the suspected vasospasm reaction should be ruled out before receiving additional rizatriptan benzoate tablets doses. Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT 1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT 1 agonists have not been clearly established.
Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or a combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
Serotonin Syndrome
Serotonin syndrome may occur with triptans, including rizatriptan benzoate tablets particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.5) ] . Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms can occur within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Rizatriptan benzoate tablets treatment should be discontinued if serotonin syndrome is suspected [see Drug Interactions (7.4) and Patient Counseling Information (17) ] .
Increase in Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients with and without a history of hypertension receiving 5-HT 1 agonists, including rizatriptan benzoate tablets. In healthy young adult male and female patients who received maximal doses of rizatriptan benzoate tablets (10 mg every 2 hours for 3 doses), slight increases in blood pressure (approximately 2-3 mmHg) were observed. Rizatriptan benzoate tablets is contraindicated in patients with uncontrolled hypertension [see Contraindications (4) ] .
ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina [see Warnings and Precautions (5.1) ] .
- Arrhythmias [see Warnings and Precautions (5.2) ] .
- Chest, Throat, Neck and/or Jaw Pain/Tightness/Pressure [see Warnings and Precautions (5.3) ] .
- Cerebrovascular Events [see Warnings and Precautions (5.4) ] .
- Other Vasospasm Reactions [see Warnings and Precautions (5.5) ] .
- Medication Overuse Headache [see Warnings and Precautions (5.6) ] .
- Serotonin Syndrome [see Warnings and Precautions (5.7) ] .
- Increase in Blood Pressure [see Warnings and Precautions (5.8) ] .
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. Adults Incidence in Controlled Clinical Trials Adverse reactions to rizatriptan benzoate tablets were assessed in controlled clinical trials that included over 3700 adult patients who received single or multiple doses of rizatriptan benzoate tablets. The most common adverse reactions during treatment with rizatriptan benzoate tablets (≥5% in either treatment group and greater than placebo) were asthenia/fatigue, somnolence, pain/pressure sensation and dizziness. These adverse reactions appeared to be dose related. Table 1 lists the adverse reactions (incidence ≥2% and greater than placebo) after a single dose of rizatriptan benzoate tablets in adults.
| % of Patients | |||
| Adverse Reactions | Rizatriptan benzoate tablets 5 mg (N=977) | Rizatriptan benzoate tablets 10 mg (N=1167) | Placebo (N=627) |
| Atypical Sensations | 4 | 5 | 4 |
| Paresthesia | 3 | 4 | <2 |
| Pain and other Pressure Sensations | 6 | 9 | 3 |
| Chest Pain: | |||
| tightness/pressure and/or heaviness | <2 | 3 | 1 |
| Neck/throat/jaw: | |||
| pain/tightness/pressure | <2 | 2 | 1 |
| Regional Pain: | |||
| tightness/pressure and/or heaviness | <1 | 2 | 0 |
| Pain, location unspecified | 3 | 3 | <2 |
| Digestive | 9 | 13 | 8 |
| Dry Mouth | 3 | 3 | 1 |
| Nausea | 4 | 6 | 4 |
| Neurological | 14 | 20 | 11 |
| Dizziness | 4 | 9 | 5 |
| Headache | <2 | 2 | <1 |
| Somnolence | 4 | 8 | 4 |
| Other | |||
| Asthenia/fatigue | 4 | 7 | 2 |
The frequencies of adverse reactions in clinical trials did not increase when up to three doses were taken within 24 hours. Adverse reaction frequencies were also unchanged by concomitant use of drugs commonly taken for migraine prophylaxis (including propranolol), oral contraceptives, or analgesics. The incidences of adverse reactions were not affected by age or gender. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Other Events Observed in Association with the Administration of Rizatriptan benzoate tablets in Adults
In the following section, the frequencies of less commonly reported adverse events are presented that were not reported in other sections of the labeling. Because the reports include events observed in open studies, the role of rizatriptan benzoate tablets in their causation cannot be reliably determined. Furthermore, variability associated with adverse event reporting, the terminology used to describe adverse events, limit the value of the quantitative frequency estimates provided. Event frequencies are calculated as the number of patients who used rizatriptan benzoate tablets and reported an event divided by the total number of patients exposed to rizatriptan benzoate tablets (N=3716). All reported events occurred at an incidence ≥1%, or are believed to be reasonably associated with the use of the drug. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are those defined as those occurring in at least (>)1/100 patients; infrequent adverse experiences are those occurring in 1/100 to 1/1000 patients; and rare adverse experiences are those occurring in fewer than 1/1000 patients.
General: Infrequent was facial edema. Rare were syncope and edema/swelling.
Atypical Sensations: Frequent were warm sensations.
Cardiovascular: Frequent was palpitation. Infrequent were tachycardia, cold extremities, and bradycardia.
Digestive: Frequent were diarrhea and vomiting. Infrequent were dyspepsia, tongue edema and abdominal distention.
Musculoskeletal: Infrequent were muscle weakness, stiffness, myalgia and muscle cramp/spasm.
Neurological/Psychiatric: Frequent were hypoesthesia, euphoria and tremor. Infrequent were vertigo, insomnia, confusion/disorientation, gait abnormality, memory impairment, and agitation.
Respiratory: Frequent was dyspnea. Infrequent was pharyngeal edema.
Special Senses: Infrequent were blurred vision and tinnitus. Rare was eye swelling.
Skin and Skin Appendage: Frequent was flushing. Infrequent were sweating, pruritus, rash, and urticaria. Rare was erythema, hot flashes.
The adverse reaction profile seen with rizatriptan benzoate orally disintegrating tablets was similar to that seen with rizatriptan benzoate tablets.
Pediatric Patients 6 to 17 Years of Age
Incidence in Controlled Clinical Trials in Pediatric Patients
Adverse reactions to rizatriptan benzoate orally disintegrating tablets were assessed in a controlled clinical trial in the acute treatment of migraines (Study 7) that included a total of 1382 pediatric patients 6-17 years of age, of which 977 (72%) administered at least one dose of study treatment (rizatriptan benzoate orally disintegrating tablets and/or placebo) [see Clinical Studies (14.2 )]. The incidence of adverse reactions reported for pediatric patients in the acute clinical trial was similar in patients who received rizatriptan benzoate tablet to those who received placebo. The adverse reaction pattern in pediatric patients is expected to be similar to that in adults.
Other Events Observed in Association with the Administration of Rizatriptan benzoate orally disintegrating tablets in Pediatric Patients
In the following section, the frequencies of less commonly reported adverse events are presented. Because the reports include events observed in open studies, the role of rizatriptan benzoate orally disintegrating tablets in their causation cannot be reliably determined. Furthermore, variability associated with adverse event reporting, the terminology used to describe adverse events, limit the value of the quantitative frequency estimates provided.
Event frequencies are calculated as the number of pediatric patients 6 to 17 years of age who used rizatriptan benzoate orally disintegrating tablets and reported an event divided by the total number of patients exposed to rizatriptan benzoate orally disintegrating tablets (N=1068). All reported events occurred at an incidence ≥1%, or are believed to be reasonably associated with the use of the drug. Events are further classified within system organ class and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are those occurring in (>)1/100 pediatric patients; infrequent adverse experiences are those occurring in 1/100 to 1/1000 pediatric patients; and rare adverse experiences are those occurring in fewer than 1/1000 patients.
General: Frequent was fatigue.
Ear and labyrinth disorders: Infrequent was hypoacusis.
Gastrointestinal disorders: Frequent was abdominal discomfort.
Nervous system disorders: Infrequent were coordination abnormal, disturbance in attention, and presyncope.
Psychiatric disorders: Infrequent was hallucination.
Postmarketing Experience
The following section enumerates potentially important adverse events that have occurred in clinical practice and which have been reported spontaneously to various surveillance systems. The events enumerated include all except those already listed in other sections of the labeling or those too general to be informative. Because the reports cite events reported spontaneously from worldwide postmarketing experience, frequency of events and the role of rizatriptan benzoate tablets in their causation cannot be reliably determined. Neurological/Psychiatric: Seizure. General: Allergic conditions including anaphylaxis/anaphylactoid reaction, angioedema, wheezing, and toxic epidermal necrolysis [see Contraindications (4) ] . Special Senses: Dysgeusia.
DRUG INTERACTIONS
Propranolol
The dose of rizatriptan benzoate tablets should be adjusted in propranolol-treated patients, as propranolol has been shown to increase the plasma AUC of rizatriptan by 70% [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3) ] .
Ergot-Containing Drugs
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and rizatriptan benzoate tablets within 24 hours is contraindicated [see Contraindications (4) ] .
Other 5-HT 1 Agonists
Because their vasospastic effects may be additive, co-administration of rizatriptan benzoate tablets and other 5-HT 1 agonists within 24 hours of each other is contraindicated [see Contraindications (4) ] .
SSRIs/SNRIs and Serotonin Syndrome
Cases of serotonin syndrome have been reported during co-administration of triptans and selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) [see Warnings and Precautions (5.7) ] .
Monoamine Oxidase Inhibitors
Rizatriptan benzoate tablets is contraindicated in patients taking MAO-A inhibitors and non-selective MAO inhibitors. A specific MAO-A inhibitor increased the systemic exposure of rizatriptan and its metabolite [see Contraindications (4) and Clinical Pharmacology (12.3) ] .
DESCRIPTION
Rizatriptan benzoate tablet, USP contains rizatriptan benzoate, a selective 5-hydroxytryptamine 1B/1D (5-HT 1B/1D ) receptor agonist. Rizatriptan benzoate is described chemically as: N,N -dimethyl-5-(1 H -1,2,4-triazol-1-ylmethyl)-1 H -indole-3-ethanamine monobenzoate and its structural formula is:
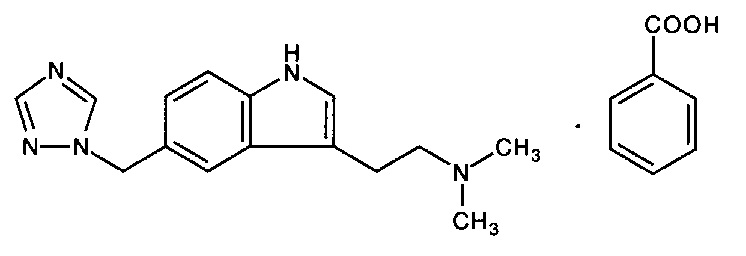
Its empirical formula is C 15 H 19 N 5 •C 7 H 6 O 2 , representing a molecular weight of the free base of 269.4. Rizatriptan benzoate is a white to off-white, crystalline solid that is soluble in water at about 42 mg per mL (expressed as free base) at 25°C. Rizatriptan benzoate Tablets, USP are available for oral administration in strengths of 5 and 10 mg (corresponding to 7.265 mg or 14.53 mg of the benzoate salt, respectively). Each compressed tablet contains the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, pregelatinized starch (maize), and magnesium stearate.
CLINICAL PHARMACOLOGY
Mechanism of Action
Rizatriptan binds with high affinity to human cloned 5-HT 1B/1D receptors. Rizatriptan benzoate tablets presumably exerts its therapeutic effects in the treatment of migraine headache by binding to 5-HT 1B/1D receptors located on intracranial blood vessels and sensory nerves of the trigeminal system.
Pharmacokinetics
Absorption Rizatriptan is completely absorbed following oral administration. The mean oral absolute bioavailability of the rizatriptan benzoate tablet is about 45%, and mean peak plasma concentrations (C max ) are reached in approximately 1-1.5 hours (T max ). The presence of a migraine headache did not appear to affect the absorption or pharmacokinetics of rizatriptan. Food has no significant effect on the bioavailability of rizatriptan but delays the time to reach peak concentration by an hour. In clinical trials, rizatriptan benzoate tablets was administered without regard to food.
The bioavailability and C max of rizatriptan were similar following administration of rizatriptan benzoate tablets and rizatriptan benzoate orally disintegrating tablets, but the rate of absorption is somewhat slower with rizatriptan benzoate orally disintegrating tablets, with T max delayed by up to 0.7 hour. AUC of rizatriptan is approximately 30% higher in females than in males. No accumulation occurred on multiple dosing.
Distribution The mean volume of distribution is approximately 140 liters in male subjects and 110 liters in female subjects. Rizatriptan is minimally bound (14%) to plasma proteins. Metabolism The primary route of rizatriptan metabolism is via oxidative deamination by monoamine oxidase-A (MAO-A) to the indole acetic acid metabolite, which is not active at the 5-HT 1B/1D receptor. N-monodesmethyl-rizatriptan, a metabolite with activity similar to that of parent compound at the 5-HT 1B/1D receptor, is formed to a minor degree. Plasma concentrations of N-monodesmethyl-rizatriptan are approximately 14% of those of parent compound, and it is eliminated at a similar rate. Other minor metabolites, the N-oxide, the 6-hydroxy compound, and the sulfate conjugate of the 6-hydroxy metabolite are not active at the 5-HT 1B/1D receptor. Elimination The total radioactivity of the administered dose recovered over 120 hours in urine and feces was 82% and 12%, respectively, following a single 10-mg oral administration of 14 C-rizatriptan. Following oral administration of 14 C-rizatriptan, rizatriptan accounted for about 17% of circulating plasma radioactivity. Approximately 14% of an oral dose is excreted in urine as unchanged rizatriptan while 51% is excreted as indole acetic acid metabolite, indicating substantial first pass metabolism. The plasma half-life of rizatriptan in males and females averages 2-3 hours. Cytochrome P450 Isoforms Rizatriptan is not an inhibitor of the activities of human liver cytochrome P450 isoforms 3A4/5, 1A2, 2C9, 2C19, or 2E1; rizatriptan is a competitive inhibitor (K i =1400 nM) of cytochrome P450 2D6, but only at high, clinically irrelevant concentrations. Special Populations Geriatric : Rizatriptan pharmacokinetics in healthy elderly non-migraineur volunteers (age 65-77 years) were similar to those in younger non-migraineur volunteers (age 18-45 years).
Pediatric: The pharmacokinetics of rizatriptan was determined in pediatric migraineurs 6 to 17 years of age. Exposures following single dose administration of 5 mg rizatriptan benzoate orally disintegrating tablets to pediatric patients weighing 20-39 kg (44-87 lb) or 10 mg rizatriptan benzoate orally disintegrating tablets to pediatric patients weighing ≥40 kg (88 lb) were similar to those observed following single dose administration of 10 mg rizatriptan benzoate orally disintegrating tablets to adults.
Gender : The mean AUC 0-∞ and C max of rizatriptan (10 mg orally) were about 30% and 11% higher in females as compared to males, respectively, while T max occurred at approximately the same time. Hepatic impairment : Following oral administration in patients with hepatic impairment caused by mild to moderate alcoholic cirrhosis of the liver, plasma concentrations of rizatriptan were similar in patients with mild hepatic insufficiency compared to a control group of subjects with normal hepatic function; plasma concentrations of rizatriptan were approximately 30% greater in patients with moderate hepatic insufficiency. Renal impairment : In patients with renal impairment (creatinine clearance 10-60 mL/min/1.73 m 2 ), the AUC 0-∞ of rizatriptan was not significantly different from that in subjects with normal renal function. In hemodialysis patients, (creatinine clearance <2 mL/min/1.73 m 2 ), however, the AUC for rizatriptan was approximately 44% greater than that in patients with normal renal function. Race : Pharmacokinetic data revealed no significant differences between African American and Caucasian subjects. Drug Interactions [See also Drug Interactions (7) .] Monoamine oxidase inhibitors : Rizatriptan is principally metabolized via monoamine oxidase, ‘A’ subtype (MAO-A). Plasma concentrations of rizatriptan may be increased by drugs that are selective MAO-A inhibitors (e.g., moclobemide) or nonselective MAO inhibitors [type A and B] (e.g., isocarboxazid, phenelzine, tranylcypromine, and pargyline). In a drug interaction study, when rizatriptan benzoate tablets 10 mg was administered to subjects (n=12) receiving concomitant therapy with the selective, reversible MAO-A inhibitor, moclobemide 150 mg t.i.d., there were mean increases in rizatriptan AUC and C max of 119% and 41% respectively; and the AUC of the active N-monodesmethyl metabolite of rizatriptan was increased more than 400%. The interaction would be expected to be greater with irreversible MAO inhibitors. No pharmacokinetic interaction is anticipated in patients receiving selective MAO-B inhibitors [see Contraindications (4) and Drug Interactions (7.5) ]. Propranolol : In a study of concurrent administration of propranolol 240 mg/day and a single dose of rizatriptan 10 mg in healthy adult subjects (n=11), mean plasma AUC for rizatriptan was increased by 70% during propranolol administration, and a four-fold increase was observed in one subject. The AUC of the active N-monodesmethyl metabolite of rizatriptan was not affected by propranolol [see Dosage and Administration (2.4) and Drug Interactions (7.1) ]. Nadolol/Metoprolol : In a drug interactions study, effects of multiple doses of nadolol 80 mg or metoprolol 100 mg every 12 hours on the pharmacokinetics of a single dose of 10 mg rizatriptan were evaluated in healthy subjects (n=12). No pharmacokinetic interactions were observed. Paroxetine : In a study of the interaction between the selective serotonin reuptake inhibitor (SSRI) paroxetine 20 mg/day for two weeks and a single dose of rizatriptan benzoate tablets 10 mg in healthy subjects (n=12), neither the plasma concentrations of rizatriptan nor its safety profile were affected by paroxetine [see Warnings and Precautions (5.7) , Drug Interactions (7.4) , and Patient Counseling Information (17) ] . Oral contraceptives : In a study of concurrent administration of an oral contraceptive during 6 days of administration of rizatriptan benzoate tablets (10 mg/day to 30 mg/day) in healthy female volunteers (n=18), rizatriptan did not affect plasma concentrations of ethinyl estradiol or norethindrone.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis Oral carcinogenicity studies of rizatriptan were conducted in mice (100 weeks) and rats (106 weeks) at doses of up to 125 mg/kg/day. Plasma exposures (AUC) at the highest dose tested were approximately 150 (mice) and 240 times (rats) that in humans at the maximum recommended human dose (MRHD) of 30 mg/day. There was no evidence of an increase in tumor incidence related to rizatriptan in either species. Mutagenesis Rizatriptan was neither mutagenic nor clastogenic in a battery of in vitro and in vivo genetic toxicity studies, including: the microbial mutagenesis (Ames) assay, in vitro mammalian cell mutagenesis and chromosomal aberration assays, and the in vivo chromosomal aberration assay in mouse. Impairment of Fertility Oral administration of rizatriptan (0, 2, 10, or 100 mg/kg/day) to female rats prior to and during mating and continuing throughout gestation and lactation resulted in no effect on fertility; however, altered estrous cyclicity and delays in time to mating were observed at the highest dose tested. Plasma exposure at the no-effect dose (10 mg/kg/day) for reproductive toxicity was approximately 15 times that in humans at the MRHD. Oral administration of rizatriptan (0, 5, 35, or 250 mg/kg/day) to male rats prior to and during mating resulted in no impairment of fertility or reproductive performance. Plasma exposure (AUC) at the highest dose tested was approximately 550 times that in humans at the MRHD.
CLINICAL STUDIES
Adults
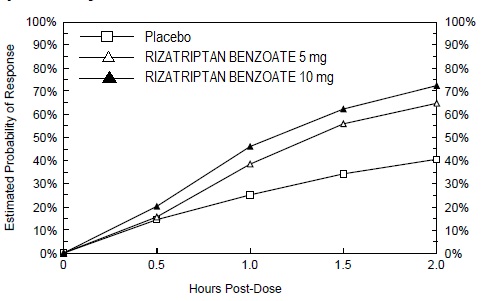
The efficacy of rizatriptan benzoate tablets was established in four multicenter, randomized, placebo-controlled trials. Patients enrolled in these studies were primarily female (84%) and Caucasian (88%), with a mean age of 40 years (range of 18 to 71). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction of moderate or severe headache pain to no or mild headache pain, was assessed for up to 2 hours (Study 1) or up to 4 hours after dosing (Studies 2, 3 and 4). Associated symptoms of nausea, photophobia, and phonophobia and maintenance of response up to 24 hours post-dose were evaluated. A second dose of rizatriptan benzoate tablets was allowed 2 to 24 hours after dosing for treatment of recurrent headache in Studies 1 and 2. Additional analgesics and/or antiemetics were allowed 2 hours after initial treatment for rescue in all four studies. In all studies, the percentage of patients achieving headache response 2 hours after treatment was significantly greater in patients who received either rizatriptan benzoate tablets 5 or 10 mg compared to those who received placebo. In a separate study, doses of 2.5 mg were not different from placebo. Doses greater than 10 mg were associated with an increased incidence of adverse effects. The results from the four controlled studies are summarized in Table 2.
| •p-value <0.05 in comparison with placebo. † p-value <0.05 in comparison with 5 mg. ‡ Results for initial headache only. | |||
| Study | Placebo | Rizatriptan Benzoate Tablets 5 mg | Rizatriptan Benzoate Tablets 10 mg |
| 1 | 35% (n=304) | 62%• (n=458) | 71%• ,† (n=456) |
| 2 ‡ | 37% (n=82) | -- | 77%• (n=320) |
| 3 | 23% (n=80) | 63%• (n=352) | -- |
| 4 | 40% (n=159) | 60%• (n=164) | 67%• (n=385) |
Comparisons of drug performance based upon results obtained in different clinical trials may not be reliable. Because studies are conducted at different times, with different samples of patients, by different investigators, employing different criteria and/or different interpretations of the same criteria, under different conditions (dose, dosing regimen, etc.), quantitative estimates of treatment response and the timing of response may be expected to vary considerably from study to study. The estimated probability of achieving an initial headache response within 2 hours following treatment in pooled Studies 1, 2, 3, and 4 is depicted in Figure 1. Figure 1: Estimated Probability of Achieving an Initial Headache Response by 2 Hours in Pooled Studies 1, 2, 3, and 4 • • Figure 1 shows the Kaplan-Meier plot of the probability over time of obtaining headache response (no or mild pain) following treatment with rizatriptan benzoate tablets or placebo. The averages displayed are based on pooled data from 4 placebo-controlled, outpatient trials providing evidence of efficacy (Studies 1, 2, 3, and 4). Patients taking additional treatment or not achieving headache response prior to 2 hours were censored at 2 hours. For patients with migraine-associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of rizatriptan benzoate tablets compared to placebo. Two to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain response in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure 2: Estimated Probability of Patients Taking a Second Dose of Rizatriptan benzoate Tablets, USP or Other Medication for Migraines Over the 24 Hours Following the Initial Dose of Study Treatment in Pooled Studies 1, 2, 3, and 4 •
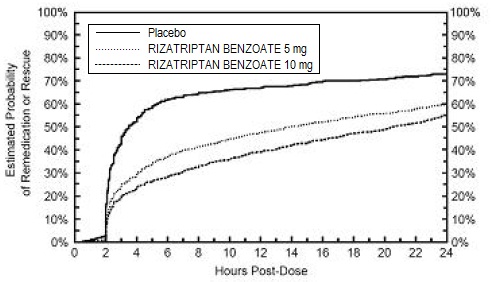
• This Kaplan-Meier plot is based on data obtained in 4 placebo-controlled outpatient clinical trials (Studies 1, 2, 3, and 4). Patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. Remedication was not allowed within 2 hours post- dose. Efficacy was unaffected by the presence of aura; by the gender, or age of the patient; or by concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants) or oral contraceptives. In two additional similar studies, efficacy was unaffected by relationship to menses. There were insufficient data to assess the impact of race on efficacy.
Pediatric Patients 6 to 17 Years of Age
The efficacy of rizatriptan benzoate orally disintegrating tablets in pediatric patients 6 to 17 years was evaluated in a multicenter, randomized, double-blind, placebo-controlled, parallel group clinical trial (Study 7). Patients had to have at least a 6-month history of migraine attacks (with or without aura) usually lasting 3 hours or more (when untreated). The patient population was historically non-responsive to NSAIDs and acetaminophen therapy.
Patients were instructed to treat a single migraine attack with headache pain of moderate to severe intensity. The treatment phase of the study had two stages. Stage 1 was used to identify placebo non-responders, who then entered into Stage 2, in which patients were randomized to rizatriptan benzoate orally disintegrating tablets or placebo. Using a weight-based dosing strategy, patients 20 kg to <40 kg (44 lb to <88 lb) received rizatriptan benzoate orally disintegrating tablets 5 mg or placebo, and patients ≥40 kg (88 lb) received rizatriptan benzoate orally disintegrating tablets10 mg or placebo.
The mean age for the studied patient population was 13 years. Sixty-one percent of the patients were Caucasian, and fifty-six percent of the patients were female. The percentage of patients achieving the primary efficacy endpoint of no headache pain at 2 hours after treatment was significantly greater in patients who received rizatriptan benzoate orally disintegrating tablets, compared with those who received placebo (33% vs. 24%). Study 7 results are summarized in Table 4.
Table 4: Response Rates 2 Hours Following Treatment of Initial Headache in Pediatric Patients 6 to 17 Years of Age in Study 7
| Endpoint | Placebo | Rizatriptan benzoate orally disintegrating tablets | p-Value |
| No headache pain at 2 hours post-dose | 24% (n/m=94/388) | 33% (n/m=126/382) | 0.01 |
n = Number of evaluable patients with no headache pain at 2 hours post-dose.
m = Number of evaluable patients in population.
The observed percentage of pediatric patients achieving no headache pain within 2 hours following initial treatment with rizatriptan benzoate orally disintegrating tablets is shown in Figure 5.
Figure 5: Observed Percentage of Patients Reporting No Headache Pain by 2 Hours Post-Dose in Study 7 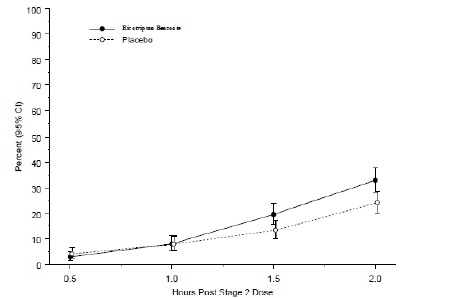 The prevalence of the exploratory endpoints of absence of migraine-associated symptoms (nausea, photophobia, and phonophobia) at 2 hours after taking the dose was not statistically significantly different between patients who received rizatriptan benzoate orally disintegrating tablets and those who received placebo.
The prevalence of the exploratory endpoints of absence of migraine-associated symptoms (nausea, photophobia, and phonophobia) at 2 hours after taking the dose was not statistically significantly different between patients who received rizatriptan benzoate orally disintegrating tablets and those who received placebo.
HOW SUPPLIED/STORAGE AND HANDLING
Rizatriptan benzoate tablets, USP 5 mg, are white to off-white, capsule-shaped, compressed tablets debossed “ RZT” on one side and “5” on other side. They are supplied as follows: NDC 67877-261-30, bottle of 30 tablets. NDC 67877-261-01, bottle of 100 tablets. NDC 67877-261-05, bottle of 500 tablets. NDC 67877-261-18, carton of 18 tablets NDC 67877-261-25, carton of 12 tablets Rizatriptan benzoate tablets, USP 10 mg, are white to off-white, capsule-shaped, compressed tablets debossed “RZT” on one side and “10” on other side. They are supplied as follows: NDC 67877-262-30, bottle of 30 tablets. NDC 67877-262-01, bottle of 100 tablets. NDC 67877-262-05, bottle of 500 tablets. NDC 67877-262-18, carton of 18 tablets NDC 67877-262-25, carton of 12 tablets Storage Store rizatriptan benzoate tablets, USP at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mechanism of Action
Rizatriptan binds with high affinity to human cloned 5-HT 1B/1D receptors. Rizatriptan benzoate tablets presumably exerts its therapeutic effects in the treatment of migraine headache by binding to 5-HT 1B/1D receptors located on intracranial blood vessels and sensory nerves of the trigeminal system.