Rocuronium Bromide
Rocuronium Bromide Prescribing Information
Rocuronium Bromide Injection is indicated for inpatients and outpatients as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Rocuronium Bromide Injection is available as
- 5 mL multiple dose vials containing 50 mg Rocuronium Bromide Injection (10 mg/mL)
- 10 mL multiple dose vials containing 100 mg Rocuronium Bromide Injection (10 mg/mL)
Rocuronium Bromide is contraindicated in patients known to have hypersensitivity (e.g., anaphylaxis) to rocuronium bromide or other neuromuscular blocking agents
Severe anaphylactic reactions to neuromuscular blocking agents, including Rocuronium Bromide, have been reported. These reactions have, in some cases (including cases with Rocuronium Bromide), been life threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those patients who have had previous anaphylactic reactions to other neuromuscular blocking agents, since cross-reactivity between neuromuscular blocking agents, both depolarizing and non depolarizing, has been reported.
In clinical trials, the most common adverse reactions (2%) are transient hypotension and hypertension.
The following adverse reactions are described, or described in greater detail, in other sections:
- Anaphylaxis [see Warnings and Precautions ()]
5.2 AnaphylaxisSevere anaphylactic reactions to neuromuscular blocking agents, including Rocuronium Bromide, have been reported. These reactions have, in some cases (including cases with Rocuronium Bromide), been life threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those patients who have had previous anaphylactic reactions to other neuromuscular blocking agents, since cross-reactivity between neuromuscular blocking agents, both depolarizing and non depolarizing, has been reported.
- Residual paralysis [see Warnings and Precautions ()]
5.5 Residual ParalysisIn order to prevent complications resulting from residual paralysis, it is recommended to extubate only after the patient has recovered sufficiently from neuromuscular block. Geriatric patients (65 years or older) may be at increased risk for residual neuromuscular block. Other factors which could cause residual paralysis after extubation in the post-operative phase (such as drug interactions or patient condition) should also be considered. If not used as part of standard clinical practice the use of a reversal agent should be considered, especially in those cases where residual paralysis is more likely to occur.
- Myopathy [see Warnings and Precautions ()]
5.6 Long-Term Use in an Intensive Care UnitRocuronium Bromide has not been studied for long-term use in the intensive care unit (ICU). As with other nondepolarizing neuromuscular blocking drugs, apparent tolerance to Rocuronium Bromide may develop during chronic administration in the ICU. While the mechanism for development of this resistance is not known, receptor up-regulation may be a contributing factor.
It is strongly recommended that neuromuscular transmission be monitored continuously during administration and recovery with the help of a nerve stimulator. Additional doses of Rocuronium Bromide or any other neuromuscular blocking agent should not be given until there is a definite response (one twitch of the train-of-four) to nerve stimulation.Prolonged paralysis and/or skeletal muscle weakness may be noted during initial attempts to wean from the ventilator patients who have chronically received neuromuscular blocking drugs in the ICU.Myopathy after long-term administration of other nondepolarizing neuromuscular blocking agents in the ICU alone or in combination with corticosteroid therapy has been reported. Therefore, for patients receiving both neuromuscular blocking agents and corticosteroids, the period of use of the neuromuscular blocking agent should be limited as much as possible and only used in the setting where in the opinion of the prescribing physician, the specific advantages of the drug outweigh the risk.
- Increased pulmonary vascular resistance [see Warnings and Precautions ()]
5.12 Increase in Pulmonary Vascular ResistanceRocuronium Bromide may be associated with increased pulmonary vascular resistance, so caution is appropriate in patients with pulmonary hypertension or valvular heart disease
[see Clinical Studies ].
Rocuronium Bromide Injection is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration. Rocuronium bromide is chemically designated as 1-[17β-(acetyloxy)-3α-hydroxy-2β-(4-morpholinyl)-5α-androstan-16β-yl]-1-(2-propenyl)pyrrolidinium bromide.
The structural formula is:
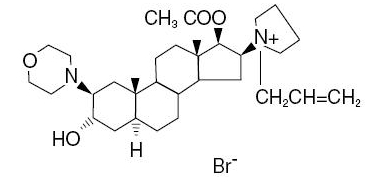
The chemical formula is C32H53BrN2O4 with a molecular weight of 609.70. The partition coefficient of rocuronium bromide in n-octanol/water is 0.5 at 20°C.
Rocuronium bromide is supplied as a sterile, nonpyrogenic, isotonic solution that is clear, colorless to yellow/orange, for intravenous injection only. Each mL contains 10 mg rocuronium bromide and 2 mg sodium acetate. The aqueous solution is adjusted to isotonicity with sodium chloride and to a pH of 4 with acetic acid and/or sodium hydroxide.