Rocuronium Bromide - Rocuronium Bromide injection, Solution
(Rocuronium Bromide)Rocuronium Bromide - Rocuronium Bromide injection, Solution Prescribing Information
Rocuronium Bromide Injection is indicated for inpatients and outpatients as an adjunct to general anesthesia to facilitate both rapid sequence and routine tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Rocuronium Bromide Injection is available as
- 50 mg/5 mL (10 mg/mL), multiple dose vials
Rocuronium Bromide Injection is contraindicated in patients known to have hypersensitivity (e.g., anaphylaxis) to rocuronium bromide or other neuromuscular blocking agents
In clinical trials, the most common adverse reactions (2%) are transient hypotension and hypertension.
The following adverse reactions are described, or described in greater detail, in other sections:
Anaphylaxis [
Residual paralysis [
Myopathy [
Increased pulmonary vascular resistance [
Rocuronium Bromide Injection is a nondepolarizing neuromuscular blocking agent with a rapid to intermediate onset depending on dose and intermediate duration. Rocuronium bromide is chemically designated as 1- [17β-(acetyloxy)-3α-hydroxy-2β-(4-morpholinyl)-5α-androstan-16β-yl]-1-(2-propenyl)pyrrolidinium bromide.
The structural formula is:
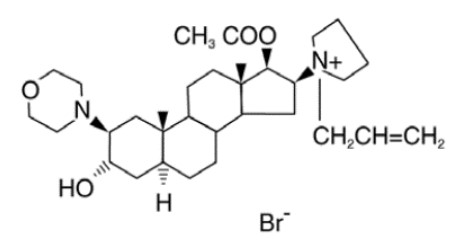
The chemical formula is C
32H
53BrN
2O
4with a molecular weight of 609.70. The partition coefficient of rocuronium bromide in n-octanol/water is 0.5 at 20°C.
Rocuronium Bromide Injection is supplied as a sterile, nonpyrogenic, isotonic solution that is clear, colorless to yellow/orange, for intravenous injection only. Each mL contains 10 mg rocuronium bromide and 2 mg sodium acetate trihydrate. The aqueous solution is adjusted to isotonicity with sodium chloride and to a pH of 4 with Glacial acetic acid and/or sodium hydroxide.
In US clinical studies, a total of 1137 patients received Rocuronium Bromide Injection, including 176 pediatric, 140 geriatric, 55 obstetric, and 766 other adults. Most patients (90%) were ASA physical status I or II, about 9% were ASA III, and 10 patients (undergoing coronary artery bypass grafting or valvular surgery) were ASA IV. In European clinical studies, a total of 1394 patients received Rocuronium Bromide Injection, including 52 pediatric, 128 geriatric (65 years or greater), and 1214 other adults.