Rosuvastain Calcium
Rosuvastain Calcium Prescribing Information
Rosuvastatin tablets are indicated:
- To reduce the risk of stroke, myocardial infarction, and arterial revascularization procedures in adults without established coronary heart disease who are at increased risk of cardiovascular (CV) disease based on age, hsCRP ≥2 mg/L, and at least one additional CV risk factor.
- As an adjunct to diet to:
- Reduce LDL-C in adults with primary hyperlipidemia.
- Reduce low-density lipoprotein cholesterol (LDL-C) and slow the progression of atherosclerosis in adults.
- Reduce LDL-C in adults and pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH).
- As an adjunct to other LDL-C-lowering therapies, or alone if such treatments are unavailable, to reduce LDL-C in adults and pediatric patients aged 7 years and older with homozygous familial hypercholesterolemia (HoFH).
- As an adjunct to diet for the treatment of adults with:
- Primary dysbetalipoproteinemia.
- Hypertriglyceridemia.
Rosuvastatin Tablets, USP:
5 mg: White to off-white, round tablets, debossed with
10 mg: White to off-white, round tablets, debossed with
20 mg: White to off-white, round tablets, debossed with
40 mg: White to off-white, oval shaped tablets, debossed with
Rosuvastatin tablets are contraindicated in the following conditions:
- Acute liver failure or decompensated cirrhosis[see].
5.3 Hepatic DysfunctionIncreases in serum transaminases have been reported with use of rosuvastatin tablets
[see Adverse Reactions (6.1)].In most cases, these changes appeared soon after initiation, were transient, were not accompanied by symptoms, and resolved or improved on continued therapy or after a brief interruption in therapy. In a pooled analysis of placebo-controlled trials, increases in serum transaminases to more than three times the ULN occurred in 1.1% of patients taking rosuvastatin tablets versus 0.5% of patients treated with placebo. Marked persistent increases of hepatic transaminases have also occurred with rosuvastatin tablets. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including rosuvastatin tablets.Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury
[see Use in Specific Populations (8.7)].Consider liver enzyme testing before rosuvastatin tablets initiation and when clinically indicated thereafter. Rosuvastatin tablets are contraindicated in patients with acute liver failure or decompensated cirrhosis
[see Contraindications (4)].If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue rosuvastatin. - Hypersensitivity to rosuvastatin or any excipients in rosuvastatin tablets. Hypersensitivity reactions including rash, pruritus, urticaria, and angioedema have been reported with rosuvastatin tablets[see.]
6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions reported in ≥2% of patients in placebo-controlled clinical studies and at a rate greater than placebo are shown in Table 2. These studies had a treatment duration of up to 12 weeks.
Table 2: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin Tablets and > Placebo in Placebo-Controlled TrialsAdverse ReactionsPlaceboN=382%Rosuvastatin5 mg
N=291%Rosuvastatin10 mg
N=283%Rosuvastatin20 mg
N=64%Rosuvastatin40 mg
N=106%Total Rosuvastatin5 mg to 40 mg N=744%Headache
5.0
5.5
4.9
3.1
8.5
5.5
Nausea
3.1
3.8
3.5
6.3
0
3.4
Myalgia
1.3
3.1
2.1
6.3
1.9
2.8
Asthenia
2.6
2.4
3.2
4.7
0.9
2.7
Constipation
2.4
2.1
2.1
4.7
2.8
2.4
Other adverse reactions reported in clinical studies were abdominal pain, dizziness, hypersensitivity (including rash, pruritus, urticaria, and angioedema) and pancreatitis. The following laboratory abnormalities have also been reported: dipstick-positive proteinuria and microscopic hematuria; elevated creatine phosphokinase, transaminases, glucose, glutamyl transpeptidase, alkaline phosphatase, and bilirubin; and thyroid function abnormalities.
In the METEOR study, patients were treated with rosuvastatin 40 mg (n=700) or placebo (n=281) with a mean treatment duration of 1.7 years. Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 3.
Table 3: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin and > Placebo in the METEOR TrialAdverse ReactionsPlaceboN=281%Rosuvastatin 40 mg N=700%Myalgia
12.1
12.7
Arthralgia
7.1
10.1
Headache
5.3
6.4
Dizziness
2.8
4.0
Increased CPK
0.7
2.6
Abdominal pain
1.8
2.4
ALT greater than 3x ULN
10.7
2.2
1Frequency recorded as abnormal laboratory value.In the JUPITER study, patients were treated with rosuvastatin 20 mg (n=8901) or placebo (n=8901) for a mean duration of 2 years. In JUPITER, there was a significantly higher frequency of diabetes mellitus reported in patients taking rosuvastatin (2.8%) versus patients taking placebo (2.3%). Mean HbA1c was significantly increased by 0.1% in rosuvastatin-treated patients compared to placebo-treated patients. The number of patients with a HbA1c >6.5% at the end of the trial was significantly higher in rosuvastatin-treated versus placebo-treated patients
[see Clinical Studies (14)].Adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 4.
Table 4: Adverse Reactions Reported in ≥2% of Patients Treated with Rosuvastatin and > Placebo in the JUPITER TrialAdverse ReactionsPlacebo N=8901%Rosuvastatin 20 mg N=8901%Myalgia
6.6
7.6
Arthralgia
3.2
3.8
Constipation
3.0
3.3
Diabetes mellitus
2.3
2.8
Nausea
2.3
2.4
Pediatric Patients with HeFHIn a 12-week controlled study in pediatric patients 10 to 17 years of age with HeFH with rosuvastatin 5 to 20 mg daily
[see Use in Specific Populations (8.4)and Clinical Studies (14)], elevations in serum CK greater than 10 x ULN were observed more frequently in rosuvastatin- treated patients compared with patients receiving placebo. Four of 130 (3%) patients treated with rosuvastatin (2 treated with 10 mg and 2 treated with 20 mg) had increased CK greater than 10 x ULN, compared to 0 of 46 patients on placebo.
The following important adverse reactions are described below and elsewhere in the labeling:
Myopathy and Rhabdomyolysis
Rosuvastatin Tablets may cause myopathy [muscle pain, tenderness, or weakness associated with elevated creatine kinase (CK)] and rhabdomyolysis. Acute kidney injury secondary to myoglobinuria and rare fatalities have occurred as a result of rhabdomyolysis with statins, including rosuvastatin tablets.
Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use with certain other drugs (including other lipid-lowering therapies), and higher rosuvastatin tablets dosage. Asian patients on rosuvastatin may be at higher risk for myopathy
The concomitant use of rosuvastatin tablets with cyclosporine or gemfibrozil is not recommended. Rosuvastatin tablets dosage modifications are recommended for patients taking certain antiviral medications, darolutamide, and regorafenib
Discontinue rosuvastatin tablets if markedly elevated CK levels occur or if myopathy is either diagnosed or suspected. Muscle symptoms and CK elevations may resolve if rosuvastatin tablets are discontinued.
Temporarily discontinue rosuvastatin tablets in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis (e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy).
Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the rosuvastatin tablets dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever.
Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use, including reports of recurrence when the same or a different statin was administered. IMNM is characterized by proximal muscle weakness and elevated serum creatine kinase that persist despite discontinuation of statin treatment; positive anti-HMG CoA reductase antibody; muscle biopsy showing necrotizing myopathy; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required. Discontinue rosuvastatin if IMNM is suspected.
Hepatic Dysfunction
Increases in serum transaminases have been reported with use of rosuvastatin tablets
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury
Consider liver enzyme testing before rosuvastatin tablets initiation and when clinically indicated thereafter. Rosuvastatin tablets are contraindicated in patients with acute liver failure or decompensated cirrhosis
Proteinuria and Hematuria
In the rosuvastatin tablets clinical trial program, dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin treated patients. These findings were more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator statins, though it was generally transient and was not associated with worsening renal function. Although the clinical significance of this finding is unknown, consider a dose reduction for patients on rosuvastatin therapy with unexplained persistent proteinuria and/or hematuria during routine urinalysis testing.
Increases in HbA1c and Fasting Serum Glucose Levels
Increases in HbA1c and fasting serum glucose levels have been reported with statins, including rosuvastatin. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus
Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices.
Rosuvastatin is a 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA)-reductase inhibitor.
The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2- [methyl(methylsulfonyl)amino] pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt with the following structural formula:
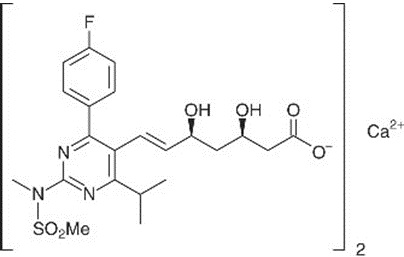
The empirical formula for rosuvastatin calcium is (C
22H
27FN
3O
6S)
2Ca and the molecular weight is 1001.14. Rosuvastatin calcium is a white amorphous powder that is sparingly soluble in water and methanol, and slightly soluble in ethanol. Rosuvastatin calcium is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.0.
Rosuvastatin Tablets, USP for oral use contain rosuvastatin 5 mg, 10 mg, 20 mg, or 40 mg (equivalent to 5.2 mg, 10.4 mg, 20.8 mg, and 41.6 mg rosuvastatin calcium) and the following inactive ingredients: butylated hydroxytoluene, lactose monohydrate, lactose anhydrous, microcrystalline cellulose, sodium citrate, crospovidone, colloidal silicon dioxide, and magnesium stearate.
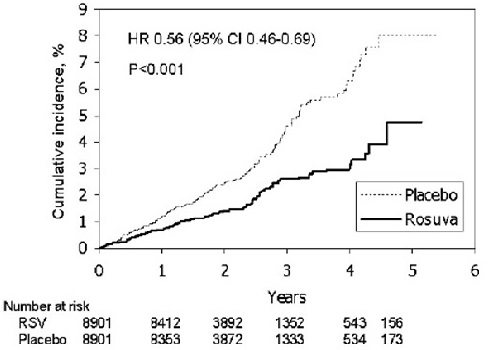
In the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study, the effect of rosuvastatin on the occurrence of major cardiovascular (CV) disease events was assessed in 17,802 men (≥50 years) and women (≥60 years) who had no clinically evident cardiovascular disease, LDL-C levels <130 mg/dL and hsCRP levels ≥2 mg/L. The study population had an estimated baseline coronary heart disease risk of 11.6% over 10 years based on the Framingham risk criteria and included a high percentage of patients with additional risk factors such as hypertension (58%), low HDL-C levels (23%), cigarette smoking (16%), or a family history of premature CHD (12%). Patients had a median baseline LDL-C of 108 mg/dL and hsCRP of 4.3 mg/L. Patients were randomly assigned to placebo (n=8901) or rosuvastatin 20 mg once daily (n=8901) and were followed for a mean duration of 2 years. The JUPITER study was stopped early by the Data Safety Monitoring Board due to meeting predefined stopping rules for efficacy in rosuvastatin-treated subjects.
The primary end point was a composite end point consisting of the time-to-first occurrence of any of the following major CV events: CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina or an arterial revascularization procedure.
Rosuvastatin significantly reduced the risk of major CV events (252 events in the placebo group vs. 142 events in the rosuvastatin group) with a statistically significant (p<0.001) relative risk reduction of 44% and absolute risk reduction of 1.2% (see Figure 1). The risk reduction for the primary end point was consistent across the following predefined subgroups: age, sex, race, smoking status, family history of premature CHD, body mass index, LDL-C, HDL-C, and hsCRP levels.
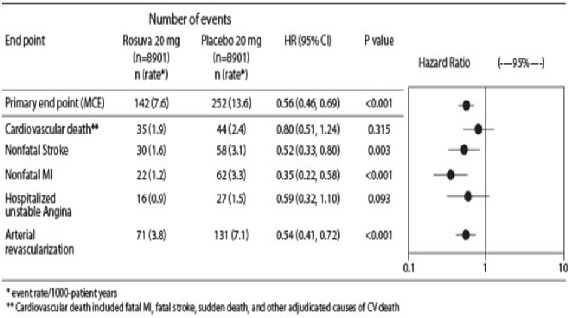
The individual components of the primary end point are presented in Figure 3. Rosuvastatin significantly reduced the risk of nonfatal myocardial infarction, nonfatal stroke, and arterial revascularization procedures. There were no significant treatment differences between the rosuvastatin and placebo groups for death due to cardiovascular causes or hospitalizations for unstable angina.
Rosuvastatin significantly reduced the risk of myocardial infarction (6 fatal events and 62 nonfatal events in placebo-treated subjects vs. 9 fatal events and 22 nonfatal events in rosuvastatin-treated subjects) and the risk of stroke (6 fatal events and 58 nonfatal events in placebo-treated subjects vs. 3 fatal events and 30 nonfatal events in rosuvastatin-treated subjects).
In a post-hoc subgroup analysis of JUPITER subjects (rosuvastatin=725, placebo=680) with a hsCRP ≥2 mg/L and no other traditional risk factors (smoking, BP ≥140/90 or taking antihypertensives, low HDL-C) other than age, after adjustment for high HDL-C, there was no significant treatment benefit with rosuvastatin treatment.
At one year, rosuvastatin increased HDL-C and reduced LDL-C, hsCRP, total cholesterol and serum triglyceride levels (p<0.001 for all versus placebo).
Rosuvastatin reduces Total-C, LDL-C, ApoB, non-HDL-C, and TG, and increases HDL-C, in adult patients with hyperlipidemia and mixed dyslipidemia.
In a multicenter, double-blind, placebo-controlled study in patients with hyperlipidemia, rosuvastatin given as a single daily dose (5 to 40 mg) for 6 weeks significantly reduced Total-C, LDL-C, non-HDL-C, and ApoB, across the dose range (Table 10).
Dose | N | Total-C | LDL-C | Non-HDL-C | ApoB | TG | HDL-C |
| Placebo | 13 | -5 | -7 | -7 | -3 | -3 | 3 |
| Rosuvastatin 5 mg | 17 | -33 | -45 | -44 | -38 | -35 | 13 |
| Rosuvastatin 10 mg | 17 | -36 | -52 | -48 | -42 | -10 | 14 |
| Rosuvastatin 20 mg | 17 | -40 | -55 | -51 | -46 | -23 | 8 |
| Rosuvastatin 40 mg | 18 | -46 | -63 | -60 | -54 | -28 | 10 |
Rosuvastatin was compared with the statins (atorvastatin, simvastatin, and pravastatin) in a multicenter, open-label, dose-ranging study of 2240 patients with hyperlipidemia or mixed dyslipidemia. After randomization, patients were treated for 6 weeks with a single daily dose of either rosuvastatin, atorvastatin, simvastatin, or pravastatin (Figure 3 and Table 11).
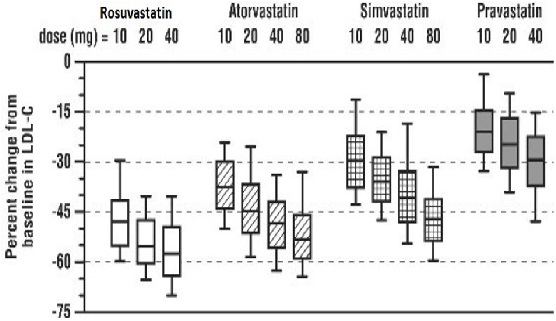
Box plots are a representation of the 25th, 50th, and 75th percentile values, with whiskers representing the 10th and 90th percentile values. Mean baseline LDL-C: 189 mg/dL
Treatment Daily Dose | ||||
Treatment | 10 mg | 20 mg | 40 mg | 80 mg |
| Rosuvastatin | -46 ² | -52 ³ | -55 ⁴ | --- |
| Atorvastatin | -37 | -43 | -48 | -51 |
| Simvastatin | -28 | -35 | -39 | -46 |
| Pravastatin | -20 | -24 | -30 | --- |
In the
The annualized rate of change from baseline for the placebo group was +0.0131 mm/year (p<0.0001). The annualized rate of change from baseline for the group treated with rosuvastatin was -0.0014 mm/year (p=0.32).
At an individual patient level in the group treated with rosuvastatin, 52.1% of patients demonstrated an absence of disease progression (defined as a negative annualized rate of change), compared to 37.7% of patients in the placebo group.
In a study of adult patients with HeFH (baseline mean LDL of 291 mg/dL), patients were randomized to rosuvastatin 20 mg or atorvastatin 20 mg. The dose was increased at 6-week intervals. Significant LDL-C reductions from baseline were seen at each dose in both treatment groups (Table 12).
| | | Rosuvastatin (n=435) LS Mean ¹ (95% CI) | Atorvastatin (n=187) LS Mean ¹ (95% CI) |
| Week 6 | 20 mg | -47% (-49%, -46%) | -38% (-40%, -36%) |
| Week 12 | 40 mg | -55% (-57%, -54%) | -47% (-49%, -45%) |
| Week 18 | 80 mg | NA | -52% (-54%, -50%) |
In a double-blind, randomized, multicenter, placebo-controlled, 12-week study, 176 (97 male and 79 female) children and adolescents with heterozygous familial hypercholesterolemia were randomized to rosuvastatin 5 mg, 10 mg or 20 mg or placebo daily. Patients ranged in age from 10 to 17 years (median age of 14 years) with approximately 30% of the patients 10 to 13 years and approximately 17%, 18%, 40%, and 25% at Tanner stages II, III, IV, and V, respectively. Females were at least 1 year postmenarche. Mean LDL-C at baseline was 233 mg/dL (range of 129 to 399). The 12-week double-blind phase was followed by a 40 week open label dose- titration phase, where all patients (n=173) received 5 mg, 10 mg or 20 mg rosuvastatin daily.
Rosuvastatin significantly reduced LDL-C (primary end point), total cholesterol and ApoB levels at each dose compared to placebo. Results are shown in Table 13 below.
Dose (mg) | N | LDL-C | HDL-C | Total-C | TG ¹ | ApoB |
| Placebo | 46 | -1% | +7% | 0% | -7% | -2% |
| 5 | 42 | -38% | +4% ² | -30% | -13% ² | -32% |
| 10 | 44 | -45% | +11% ² | -34% | -15% ² | -38% |
| 20 | 44 | -50% | +9% ² | -39% | 16% ² | -41% |
Rosuvastatin was also studied in a two-year open-label, uncontrolled, titration-to-goal trial that included 175 children and adolescents with heterozygous familial hypercholesterolemia who were 8 to 17 years old (79 boys and 96 girls). All patients had a documented genetic defect in the LDL receptor or in ApoB. Approximately 89% were White, 7% were Asian, 1% were Black, and fewer than 1% were Hispanic. Mean LDL-C at baseline was 236 mg/dL. Fifty-eight (33%) patients were prepubertal at baseline. The starting rosuvastatin dosage for all children and adolescents was 5 mg once daily. Children 8 to less than 10 years of age (n=41 at baseline) could titrate to a maximum dosage of 10 mg once daily, and children and adolescents 10 to 17 years of age could titrate to a maximum dosage of 20 mg once daily.
The reductions in LDL-C from baseline were generally consistent across age groups within the trial as well as with previous experience in both adult and pediatric controlled trials.
In an open-label, forced-titration study, HoFH patients (n=40, 8-63 years) were evaluated for their response to rosuvastatin 20 to 40 mg titrated at a 6-week interval. In the overall population, the mean LDL-C reduction from baseline was 22%. About one-third of the patients benefited from increasing their dose from 20 mg to 40 mg with further LDL-C lowering of greater than 6%. In the 27 patients with at least a 15% reduction in LDL-C, the mean LDL-C reduction was 30% (median 28% reduction). Among 13 patients with an LDL-C reduction of <15%, 3 had no change or an increase in LDL-C. Reductions in LDL-C of 15% or greater were observed in 3 of 5 patients with known receptor negative status.
Rosuvastatin was studied in a randomized, double-blind, placebo-controlled, multicenter, cross- over study in 14 pediatric patients with HoFH. The study included a 4-week dietary lead-in phase during which patients received rosuvastatin 10 mg daily, a cross-over phase that included two 6-week treatment periods with either rosuvastatin 20 mg or placebo in random order, followed by a 12-week open-label phase during which all patients received rosuvastatin 20 mg. Patients ranged in age from 7 to 15 years of age (median 11 years), 50% were male, 71% were White, 21% were Asian, 7% were Black, and no patients were of Hispanic ethnicity. Fifty percent were on apheresis therapy and 57% were taking ezetimibe. Patients who entered the study on apheresis therapy or ezetimibe continued the treatment throughout the entire study. Mean LDL-C at baseline was 416 mg/dL (range 152 to 716 mg/dL). A total of 13 patients completed both treatment periods of the randomized cross-over phase; one patient withdrew consent due to inability to have blood drawn during the cross-over phase.
Rosuvastatin 20 mg significantly reduced LDL-C, total cholesterol, ApoB, and non-HDL-C compared to placebo (Table 14).
Placebo (N=13) | Rosuvastatin 20 mg (N=13) | Percent difference (95% CI) | |
LDL-C (mg/dL) | 481 | 396 | -22.3% (-33.5, -9.1) |
Total-C (mg/dL) | 539 | 448 | -20.1% (-29.7, -9.1) |
Non-HDL-C (mg/dL) | 505 | 412 | -22.9% (-33.7, -10.3) |
ApoB (mg/dL) | 268 | 235 | -17.1% (-29.2, -2.9) |
% Difference estimates are based on transformations of the estimated mean difference in log LDL measurements between rosuvastatin and placebo using a mixed model adjusted for study period
1p=0.005,
2p=0.003,
3p=0.024
In a randomized, multicenter, double-blind crossover study, 32 adult patients (27 with є2/є2 and 4 with apo E mutation [Arg145Cys] with primary dysbetalipoproteinemia entered a 6-week dietary lead-in period on the NCEP Therapeutic Lifestyle Change (TLC) diet. Following dietary lead-in, patients were randomized to a sequence of treatments for 6 weeks each: rosuvastatin 10 mg followed by rosuvastatin 20 mg or rosuvastatin 20 mg followed by rosuvastatin 10 mg. Rosuvastatin reduced non-HDL-C (primary end point) and circulating remnant lipoprotein levels. Results are shown in the table below.
Median at Baseline (mg/dL) | Median percent change from baseline (95% CI) Rosuvastatin 10 mg | Median percent change from baseline (95% CI) Rosuvastatin 20 mg | |
| Total-C | 342.5 | -43.3 (-46.9, - 37.5) | -47.6 (-51.6,-42.8) |
| Triglycerides | 503.5 | -40.1 (-44.9, -33.6) | -43.0 (-52.5, -33.1) |
| Non-HDL-C | 294.5 | -48.2 (-56.7, -45.6) | -56.4 (-61.4, -48.5) |
| VLDL-C + IDL-C | 209.5 | -46.8 (-53.7, -39.4) | -56.2 (-67.7, -43.7) |
| LDL-C | 112.5 | -54.4 (-59.1, -47.3) | -57.3 (-59.4, -52.1) |
| HDL-C | 35.5 | 10.2 (1.9, 12.3) | 11.2 (8.3, 20.5) |
| RLP-C | 82.0 | -56.4 (-67.1, -49.0) | -64.9 (-74.0, -56.6) |
| Apo-E | 16.0 | -42.9 (-46.3, -33.3) | -42.5 (-47.1, -35.6) |
In a double-blind, placebo-controlled study in adult patients with baseline TG levels from 273 to 817 mg/dL, rosuvastatin given as a single daily dose (5 to 40 mg) over 6 weeks significantly reduced serum TG levels (Table 16).
Dose | Placebo (n=26) | Rosuvastatin 5 mg (n=25) | Rosuvastatin 10 mg (n=23) | Rosuvastatin 20 mg (n=27) | Rosuvastatin 40 mg (n=25) |
|---|---|---|---|---|---|
| Triglycerides | 1 (-40, 72) | -21 (-58, 38) | -37 (-65, 5) | -37 (-72, 11) | -43 (-80, -7) |
| Non-HDL-C | 2 (-13, 19) | -29 (-43, -8) | -49 (-59, -20) | -43 (-74, 12) | -51 (-62, -6) |
| Total-C | 1 (-13, 17) | -24 (-40, -4) | -40 (-51, -14) | -34 (-61, -11) | -40 (-51, -4) |
| LDL-C | 5 (-30, 52) | -28 (-71, 2) | -45 (-59, 7) | -31 (-66, 34) | -43 (-61, -3) |
| HDL-C | -3 (-25, 18) | 3 (-38, 33) | 8 (-8, 24) | 22 (-5, 50) | 17 (-14, 63) |