Rosyrah
(Levonorgestrel And Ethinyl Estradiol And Ethinyl Estradiol)Rosyrah Prescribing Information
Rosyrah is a combination of levonorgestrel, a progestin, and ethinyl estradiol, an estrogen, indicated for use by females of reproductive potential to prevent pregnancy. (
Rosyrah is a combination of levonorgestrel, a progestin, and ethinyl estradiol, an estrogen, indicated for use by females of reproductive potential to prevent pregnancy.
Rosyrah is indicated for use by females of reproductive age to prevent pregnancy.
If one white to off-white, light peach, bluish green tablet is missed | Take the missed tablet as soon as possible. Take the next tablet at the regular time. Continue taking one tablet a day until the pack is finished. A back-up birth control method is not required if the patient has sex. |
If two white to off-white, light peach, bluish green tablets in a row are missed | Take the two missed tablets as soon as possible, and the next two tablets the next day. Continue taking one tablet a day until the pack is finished. Use additional nonhormonal contraception (such as condoms and spermicide) until tablets have been taken for 7 days after missing tablets. |
If three or more white to off-white, light peach, bluish green tablets in a row are missed |
|
If any of the seven yellow tablets are missed |
|
Rosyrah consists of 91 tablets in the following order (
Rosyrah consists of 91 tablets in the following order :
42 white to off-white tablets containing 0.15 mg levonorgestrel and 0.02 mg ethinyl estradiol,
21 light peach tablets containing 0.15 mg of levonorgestrel and 0.025 mg ethinyl estradiol, and
21 bluish green tablets containing 0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol, and
7 yellow tablets containing 0.01 mg of ethinyl estradiol.
Rosyrah is available as round, biconvex tablets, packaged in Extended-Cycle Tablet Blister Pack, each containing a 13-week supply of tablets in the following order:
• 42 white to off-white tablets, each containing 0.15 mg of levonorgestrel and 0.02 mg ethinyl estradiol: debossed with72on one side of the tablet and plain on the other side• 21 light peach tablets containing 0.15 mg of levonorgestrel and 0.025 mg ethinyl estradiol: debossed with73on one side of the tablet and plain on the other side• 21 bluish green tablets containing 0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol: debossed with74on one side of the tablet and plain on the other side and• 7 yellow tablets containing 0.01 mg of ethinyl estradiol: debossed with65on one side of the tablet and plain on the other side
42 white to off-white tablets containing 0.15 mg levonorgestrel and 0.02 mg ethinyl estradiol,
21 light peach tablets containing 0.15 mg of levonorgestrel and 0.025 mg ethinyl estradiol, and
21 bluish green tablets containing 0.15 mg of levonorgestrel and 0.03 mg ethinyl estradiol, and
7 yellow tablets containing 0.01 mg of ethinyl estradiol.
Pregnancy: Discontinue use if pregnancy occurs. (
)8.1 PregnancyRisk SummaryThere is no use for contraception in pregnancy; therefore, levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to CHCs before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
Lactation: Advise use of another method. Levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets are not recommended for nursing mothers; may decrease milk production. (
)8.2 LactationRisk SummaryContraceptive hormones and/or metabolites are present in human milk. CHCs can reduce milk production in breastfeeding females. This reduction can occur at any time but is less likely to occur once breastfeeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breastfeeding
[See Dosage and Administration (2.1)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets and any potential adverse effects on the breastfed child from levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets or the underlying maternal condition.
A high risk of arterial or venous thrombotic diseases (
)4 CONTRAINDICATIONSA high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
Undiagnosed abnormal uterine bleeding (
)4 CONTRAINDICATIONSA high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
Breast cancer (
)4 CONTRAINDICATIONSA high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
Liver tumors or liver disease (
)4 CONTRAINDICATIONSA high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (
)4 CONTRAINDICATIONSA high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
The risk for cardiovascular disease and prevalence of risk factors for cardiovascular disease increases with age. Certain conditions, such as smoking and migraine headache without aura, that do not contraindicate COC use in younger females, are contraindications to use in women over 35 years of age
A high risk of arterial or venous thrombotic diseases
Undiagnosed abnormal uterine bleeding
Breast cancer
Liver tumors or liver disease
Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir
Rosyrah is contraindicated in females who are known to have or develop the following conditions:
A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
o Smoke, if over age 35[see Boxed Warning and Warnings and Precautions ].o Have current or history of deep vein thrombosis or pulmonary embolism[seeWarnings and Precautions ].o Have cerebrovascular disease[see Warnings and Precautions ].o Have coronary artery disease[see Warnings and Precautions ].o Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)[see Warnings and Precautions ].o Have inherited or acquired hypercoagulopathies[see Warnings and Precautions ].o Have uncontrolled hypertension or hypertension with vascular disease[see Warnings and Precautions ].o Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration[see Warnings and Precautions ].o Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches[see Warnings and Precautions ].
Current diagnosis of, or history of, breast cancer, which may be hormone sensitive
[see Warnings and Precautions (5.11)].Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis
[see Warnings and Precautions and Use in Specific Populations ].Undiagnosed abnormal uterine bleeding
[see Warnings and Precautions ].Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations
[see Warnings and Precautions ].
Stop levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets if an arterial or deep venous thromboembolic event occurs.
Stop levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Discontinue levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets during prolonged immobilization. If feasible, stop levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets no earlier than 4 weeks after delivery, in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
Before starting levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. Levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets are contraindicated in females with a high risk of arterial or venous/thromboembolic diseases
[see Contraindications ].
COCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
Levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets are contraindicated in women over 35 years of age who smoke
Use of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
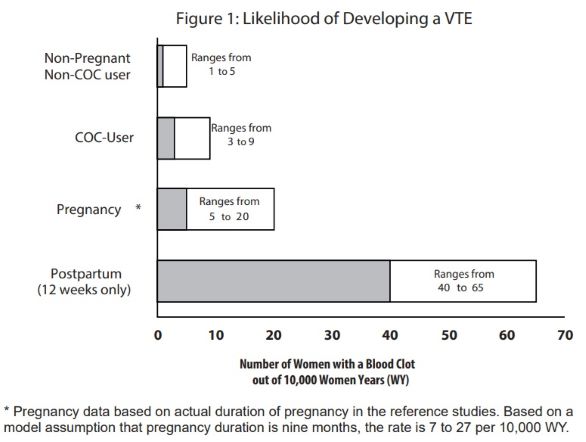
Use of levonorgestrel and ethinyl estradiol tablets and ethinyl estradiol tablets provides women with more hormonal exposure on a yearly basis than conventional monthly oral contraceptives containing the same strength synthetic estrogens and progestins (an additional 9 and 13 weeks of exposure to progestin and estrogen, respectively, per year). In the clinical trial, three cases of deep vein thrombosis were reported.

Hypertension
Diabetes
Dyslipidemia
Obesity