Sevenfact - Coagulation Factor Viia Recombinant Human Prescribing Information
Thrombosis
Serious arterial and venous thrombosis can occur with coagulation factor VIIa containing products including SEVENFACT.
The following patients may have increased risk of thrombosis with use of SEVENFACT:
- History of congenital or acquired hemophilia receiving concomitant treatment with aPCC/PCC (activated or non-activated prothrombin complex) or other hemostatic agents
- History of atherosclerotic disease, coronary artery disease, cerebrovascular disease, crush injury, septicemia, or thromboembolism.
- Monitor patients who receive SEVENFACT for the development of signs and symptoms of activation of the coagulation system or thrombosis. When there is laboratory confirmation of intravascular coagulation or presence of clinical thrombosis, reduce the dose of SEVENFACT or stop treatment, depending on the patient’s condition.
Dosage and Administration, Reconstitution (
Reconstitution
- Follow the procedures below for reconstitution of SEVENFACT.
- Calculate the amount of SEVENFACT required and select the appropriate SEVENFACT packages containing the matching pre-filled syringe of sterile Water for Injection, and the vial adapters.
- Reconstitute each vial with the pre-filled syringe provided with each vial of SEVENFACT.
The instructions below serve as a general guideline for reconstitution of SEVENFACT.
- Based on the prescribed dose, take out the number of SEVENFACT kits (each kit containing one vial of SEVENFACTpowder and one pre-filled Water for Injection diluent syringe with one vial adapter for needleless reconstitution), an infusion set (not supplied in the kit) and an alcohol swab (not supplied in the kit). Check the expiration date on the side of the box(es) for the SEVENFACT kit(s).
- Always use aseptic technique. Wash your hands with soap and water and dry them using a clean towel or air dry.
- Take out the contents of one kit and one alcohol swab. Place items on a clean surface.
- Inspect all contents of the kit. Make sure each vial has a matching colored syringe.
- Bring SEVENFACT (lyophilized powder) and the specified pre-filled syringe (diluent) to room temperature. The specified volume of diluent corresponding to the amount of SEVENFACT is as follows:
1 mg (1000 micrograms) vial + 1.1 mL Water for Injection diluent in pre-filled syringe
2 mg (2000 micrograms) vial + 2.2 mL Water for Injection diluent in pre-filled syringe
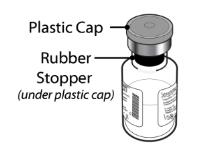
5 mg (5000 micrograms) vial + 5.2 mL Water for Injection diluent in pre-filled syringe - Remove the plastic cap from the SEVENFACT vials to expose the central portion of the rubber stopper. Cleanse the rubber stoppers with an alcohol swab and allow to dry prior to use.
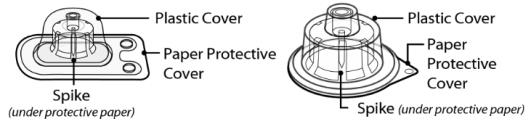
- Peel back the protective paper from the vial adapter. Do not remove the vial adapter from the package.
- Place the SEVENFACT vial on a flat surface. While holding the vial adapter package, place the vial adapter over the SEVENFACT vial and press down firmly on the package until the vial adapter spike breaks through the rubber stopper.
- Lightly squeeze the plastic cover and lift up to remove it from the vial adapter. Note: the 5 mg vial adapter may not sit flat against the vial, but it is fully functional.
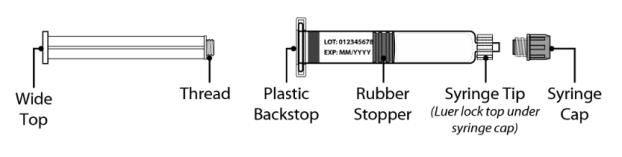
- Remove the syringe cap from the pre-filled syringe by holding the syringe body with one hand to unscrew the syringe cap (turn to the left).
- While holding the edges of the vial adapter, screw on the pre-filled syringe (turn to the right) a few turns until it starts to tighten.
- Insert the plunger rod into the syringe, then screw a few turns (turn to the right) so that the plunger rod is attached to the gray rubber stopper in the syringe.
- Push the plunger rod to slowly inject all the diluent into the vial. Keep the plunger rod pressed down and swirl the vial gently until the powder is dissolved.
- The reconstituted solution is clear to slightly opaque. All powder must be mixed with no particles floating in the liquid.
- Without withdrawing any drug back into the syringe, unscrew the syringe from the vial adapter (turn to the left) until it is completely detached.
- Withdraw the liquid drug from the vial(s), using an infusion syringe provided by the pharmacy; the syringe should be large enough to hold the prescribed dose.
- The reconstituted solution should be stored in the vial at room temperature, but can be stored between 36oF to 86oF (2oC to 30oC) for up to 4 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL SEVENFACT(1000 micrograms per mL).
06/2024
SEVENFACT is indicated for the treatment and control of bleeding episodes occurring in adults and adolescents 12 years of age and older with hemophilia A or B with inhibitors.
SEVENFACT is not indicated for the treatment of patients with congenital Factor VII deficiency.
Type of Bleeding | Dosing Regimen Recommendation |
| For Mild or Moderate bleeds | 75 mcg/kg repeated every 3 hours until hemostasis is achieved or Initial dose of 225 mcg/kg. If hemostasis is not achieved within 9 hours, additional 75 mcg/kg doses may be administered every 3 hours as needed to achieve hemostasis |
| For Severe bleeds | 225 mcg/kg, followed if necessary 6 hours later with 75 mcg/kg every 2 hours |
Consider alternative treatments if successful control of bleeding does not occur within 24 hours of the first administration of SEVENFACT.
SEVENFACT is a white to off-white lyophilized powder for reconstitution in a colorless solution for injection. It is supplied in single-dose vial sizes containing 1 mg, 2 mg or 5 mg of coagulation factor VIIa (recombinant)-jncw.
The diluent for reconstitution of SEVENFACT is supplied in single-dose prefilled glass syringes containing 1.1 mL, 2.2 mL or 5.2 mL sterile Water for Injection. It is a clear colorless solution.
After reconstitution with the appropriate volume of Water for Injection diluent, each mL of SEVENFACT contains 1 mg per mL of coagulation factor VIIa (recombinant)-jncw (1,000 micrograms per mL).
There are no adequate and well-controlled studies using SEVENFACT in pregnant women to determine whether there is a drug-associated risk. Animal studies evaluating the embryo-fetal teratogenic potential of SEVENFACT have not been conducted. It is unknown whether SEVENFACT can cause fetal harm when administered to a pregnant woman or can affect fertility.
In the U.S. general population, the estimated background risks of major birth defect and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.