Sildenafil Citrate
Sildenafil Citrate Prescribing Information
Indications and Usage (
Sildenafil for Oral Suspension is indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening
Sildenafil for Oral Suspension is a phosphodiesterase-5 (PDE-5) inhibitor indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening.
Dosage and Administration (
The recommended dosage of sildenafil for oral suspension is 20 mg three times a day.
1. Tap the bottle to loosen the powder.
2. Add 60 mL of water to the bottle.
3. Replace the cap and shake the bottle vigorously for a minimum of 30 seconds.
4. Add another 33 mL of water to the bottle.
5. Replace the cap and shake the bottle vigorously for a minimum of 30 seconds.
6. Remove cap and press the bottle adaptor into the neck of the bottle. Replace the cap on the bottle.
7. Write the expiration date of the reconstituted oral suspension on the bottle label (the expiration date of the reconstituted oral suspension is 60 days from the date of reconstitution).
Do not mix with any other medication or additional flavoring agent.
Sildenafil for Oral Suspension is indicated for the treatment of pulmonary arterial hypertension (PAH) (World Health Organization [WHO] Group I) in adults to improve exercise ability and delay clinical worsening
Patients were randomized to receive placebo (n=70) or sildenafil 20 mg (n = 69), 40 mg (n = 67) or 80 mg (n = 71) three times a day for a period of 12 weeks. They had either primary pulmonary hypertension (PPH) (63%), PAH associated with CTD (30%), or PAH following surgical repair of left-to-right congenital heart lesions (7%). The study population consisted of 25% men and 75% women with a mean age of 49 years (range: 18 to 81 years) and baseline 6-minute walk distance between 100 and 450 meters (mean 343).
The primary efficacy endpoint was the change from baseline at Week 12 (at least 4 hours after the last dose) in the 6-minute walk distance. Placebo-corrected mean increases in walk distance of 45–50 meters were observed with all doses of sildenafil. These increases were significantly different from placebo, but the sildenafil dose groups were not different from each other (see Figure 3), indicating no additional clinical benefit from doses higher than 20 mg three times a day. The improvement in walk distance was apparent after 4 weeks of treatment and was maintained at Week 8 and Week 12.
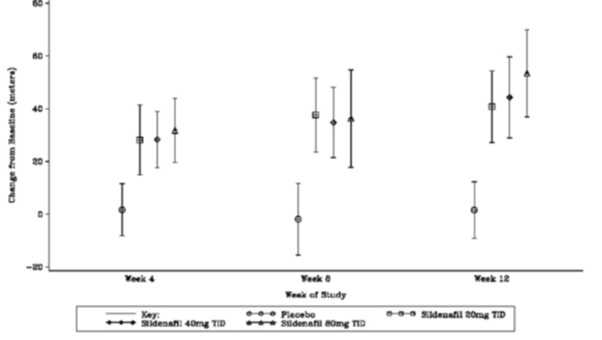
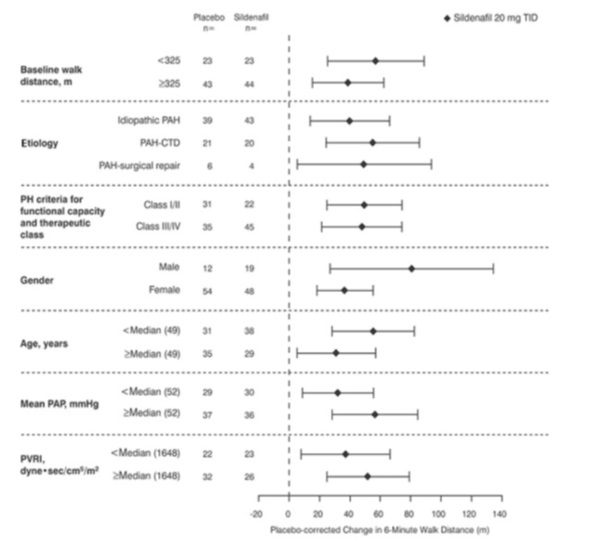
Figure 4 displays subgroup efficacy analyses in SUPER-1 for the change from baseline in 6-Minute Walk Distance at Week 12 including baseline walk distance, disease etiology, functional class, gender, age, and hemodynamic parameters.
Key
At baseline patients had PPH (80%) or PAH secondary to CTD (20%);WHO Functional Class I (1%), II (26%), III (67%), or IV (6%); and the mean age was 48 years, 80% were female, and 79% were Caucasian.
There was a statistically significant greater increase from baseline in 6-minute walk distance at Week 16 (primary endpoint) for the sildenafil group compared with the placebo group. The mean change from baseline at Week 16 (last observation carried forward) was 30 meters for the sildenafil group compared with 4 meters for the placebo group giving an adjusted treatment difference of 26 meters (95% CI: 10.8, 41.2) (p = 0.0009).
Patients on sildenafil achieved a statistically significant reduction in mPAP compared to those on placebo. A mean placebo-corrected treatment effect of -3.9 mmHg was observed in favor of sildenafil (95% CI: -5.7, -2.1) (p = 0.00003).
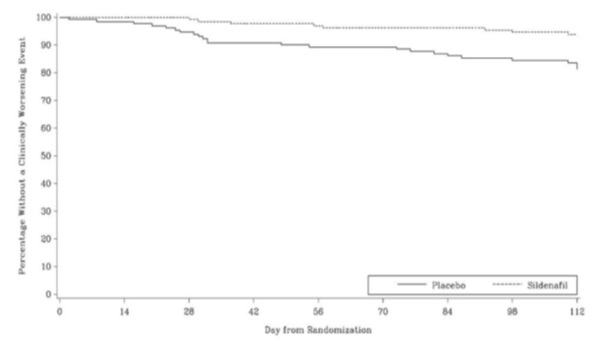
Time to clinical worsening of PAH was defined as the time from randomization to the first occurrence of a clinical worsening event (death, lung transplantation, initiation of bosentan therapy, or clinical deterioration requiring a change in epoprostenol therapy). Table 4 displays the number of patients with clinical worsening events in PACES-1. Kaplan-Meier estimates and a stratified log-rank test demonstrated that placebo-treated patients were 3 times more likely to experience a clinical worsening event than sildenafil -treated patients and that sildenafil -treated patients experienced a significant delay in time to clinical worsening versus placebo-treated patients (p = 0.0074). Kaplan-Meier plot of time to clinical worsening is presented in Figure 5.
Placebo (N = 131) | Sildenafil (N = 134) | |||
| Number of patients with clinical worsening first event | 23 | 8 | ||
First Event | All Events | First Event | All Events | |
| Death, n | 3 | 4 | 0 | 0 |
| Lung transplantation, n | 1 | 1 | 0 | 0 |
| Hospitalization due to PAH, n | 9 | 11 | 8 | 8 |
| Clinical deterioration resulting in: Change of Epoprostenol Dose, n Initiation of Bosentan, n | 9 1 | 16 1 | 0 0 | 2 0 |
| Proportion worsened 95% Confidence Interval | 0.187 (0.12 – 0.26) | 0.062 (0.02 – 0.10) | ||
Improvements in WHO Functional Class for PAH were also demonstrated in patients on sildenafil compared to placebo. More than twice as many sildenafil -treated patients (36%) as placebo-treated patients (14%) showed an improvement in at least one functional New York Heart Association (NYHA) class for PAH.
A randomized, double-blind, placebo-controlled study was conducted in 103 patients with PAH who were on bosentan therapy for a minimum of 3 months. The PAH patients included those with primary PAH and PAH associated with CTD. Patients were randomized to placebo or sildenafil (20 mg three times a day) in combination with bosentan (62.5 to 125 mg twice a day). The primary efficacy endpoint was the change from baseline at Week 12 in 6-minute walk distance (6MWD). The results indicate that there is no significant difference in mean change from baseline on 6MWD observed between sildenafil 20 mg plus bosentan and bosentan alone.



• Adults: 20 mg three times a day Dose may be increased based on symptoms and tolerability. (
The recommended dosage of sildenafil for oral suspension is 20 mg three times a day.
White to off-white powders containing 1.57 g of sildenafil citrate (equivalent to 1.12 g of sildenafil) in a bottle for reconstitution to 10 mg/mL. Following reconstitution with 93 mL of water, the total volume of the oral suspension is 112 mL. A 2-mL oral dosing syringe (with 0.5 mL and 2 mL dose markings) and a press-in bottle adaptor are also provided.
Risk Summary
Limited published data from randomized controlled trials, case-controlled trials, and case series do not report a clear association with sildenafil and major birth defects, miscarriage, or adverse maternal or fetal outcomes when sildenafil is used during pregnancy. There are risks to the mother and fetus from untreated pulmonary arterial hypertension (see Clinical Considerations). Animal reproduction studies conducted with sildenafil showed no evidence of embryo-fetal toxicity or teratogenicity at doses up to 32-and 65-times the recommended human dose (RHD) of 20 mg three times a day in rats and rabbits, respectively
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women with untreated pulmonary arterial hypertension are at risk for heart failure, stroke, preterm delivery, and maternal and fetal death.
Data
Animal Data
No evidence of teratogenicity, embryotoxicity, or fetotoxicity was observed in pregnant rats or rabbits dosed with sildenafil 200 mg/kg/day during organogenesis, a level that is, on a mg/m2 basis, 32- and 65-times, respectively, the recommended human dose (RHD) of 20 mg three times a day. In a rat pre- and postnatal development study, the no-observed-adverse-effect dose was 30 mg/kg/day (equivalent to 5-times the RHD on a mg/m2 basis).
Sildenafil is contraindicated in patients with:
· Concomitant use of organic nitrates in any form, either regularly or intermittently, because of the greater risk of hypotension
Sildenafil has vasodilatory properties, resulting in mild and transient decreases in blood pressure. Before prescribing sildenafil, carefully consider whether patients with certain underlying conditions could be adversely affected by such vasodilatory effects (e.g., patients on antihypertensive therapy or with resting hypotension [blood pressure less than 90/50], fluid depletion, severe left ventricular outflow obstruction, or autonomic dysfunction). Monitor blood pressure when co-administering blood pressure lowering drugs with sildenafil.
· Concomitant use of riociguat, a guanylate cyclase stimulator. Phosphodiesterase-5 (PDE-5) inhibitors, including sildenafil, may potentiate the hypotensive effects of riociguat.
· Known hypersensitivity to sildenafil or any component of the tablet, injection, or oral suspension. Hypersensitivity, including anaphylactic reaction, anaphylactic shock and anaphylactoid reaction, has been reported in association with the use of sildenafil.