Sodium Iodide I 123
Sodium Iodide I 123 Prescribing Information
Administration of Sodium Iodide I 123 is indicated as a diagnostic procedure to be used in evaluating thyroid function and/or morphology.
The recommended oral dose for the average patient (70 kg) is 3.7 to 14.8 MBq (100-400 µCi). The lower part of the dosage range 3.7 MBq (100 µCi) is recommended for uptake studies alone, and the higher part 14.8 MBq (400 µCi) for thyroid imaging. The determination of I 123 concentration in the thyroid gland may be initiated at six hours after administering the dose and should be measured in accordance with standardized procedures.
The patient dose should be measured by a suitable radioactive calibration system immediately prior to administration. The capsules can be utilized up to thirty (30) hours after calibration time and date. Thereafter, discard the capsules in accordance with standard safety procedures. The user should wear waterproof gloves at all times when handling the capsules or container.
To date there are no known contraindications to the use of Sodium Iodide I 123 capsules.
Although rare, reactions associated with the administration of Sodium Iodide isotopes for diagnostic use include, in decreasing order of frequency, nausea, vomiting, chest pain, tachycardia, itching skin, rash and hives.
Sodium Iodide I 123 (Na123I) for diagnostic use is supplied in capsules for oral administration. The capsules are available in strengths of 3.7 and 7.4 megabecquerels (MBq) (100 and 200 μCi) I 123 at time of calibration. Each capsule contains 0.3 µg - 3 µg Sodium Thiosulfate as a stabilizer.
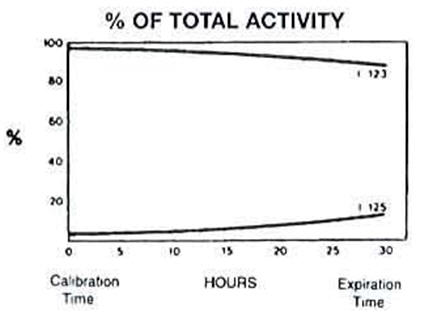
The radionuclidic composition at calibration is not less than 97.0 percent I 123, not more than 2.9 percent I 125 and not more than 0.1 percent all others (I 121 or Te 121.) The radionuclidic composition at expiration time is not less than 87.2 percent I 123, not more than 12.4 percent I 125 and not more than 0.4 percent all others. The ratio of the concentration of I 123 and I 125 changes with time. Graph 1 shows the maximum concentration of each as a function of time.
Sodium Iodide I 123 is readily absorbed from the upper gastrointestinal tract. Following absorption, the iodide is distributed primarily within the extracellular fluid of the body. It is trapped and organically bound by the thyroid and concentrated by the stomach, choroid plexus and salivary glands. It is excreted by the kidneys.
The fraction of the administered dose which is accumulated in the thyroid gland may be a measure of thyroid function in the absence of unusually high or low iodine intake or administration of certain drugs which influence iodine accumulation by the thyroid gland. Accordingly, the patient should be questioned carefully regarding previous medication and/or procedures involving radiographic media. Normal subjects can accumulate approximately 10-50% of the administered iodine dose in the thyroid gland, however, the normal and abnormal ranges are established by individual physician's criteria. The mapping (imaging) of Sodium Iodide I 123 distribution in the thyroid gland may provide useful information concerning thyroid anatomy and definition of normal and/or abnormal functioning of tissue within the gland.