Solifenacin Succinate
(Solifenacin Succiate)Solifenacin Succinate Prescribing Information
Solifenacin Succinate Tablets are indicated for the treatment of adults with overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.
Tablets:
- 5 mg: yellow, round film-coated tablets, biconvex, debossed with ‘AP28’ on one side, and plain on the other side
- 10 mg: yellow, round film-coated tablets, biconvex, debossed with ‘AP29’ on one side, and plain on the other side.
Solifenacin Succinate Tablets are contraindicated in patients:
- With urinary retention [see,]
5.2 Urinary RetentionThe use of Solifenacin Succinate Tablets, like other antimuscarinic drugs, in patients with clinically significant bladder outlet obstruction including patients with urinary retention, may result in further urinary retention and kidney injury. The use of Solifenacin Succinate Tablets is not recommended in patients with clinically significant bladder outlet obstruction and is contraindicated in patients with urinary retention
[see Contraindications (4)]. - With gastric retention [see,]
5.3 Gastrointestinal DisordersThe use of Solifenacin Succinate Tablets, like other antimuscarinic drugs, in patients with conditions associated with decreased gastrointestinal motility may result in further decreased gastrointestinal motility. Solifenacin Succinate Tablets are contraindicated in patients with gastric retention
[see Contraindications (4)]. The use of Solifenacin Succinate Tablets is not recommended in patients with conditions associated with decreased gastrointestinal motility. - With uncontrolled narrow-angle glaucoma [see, and]
5.5 Controlled Narrow-Angle GlaucomaSolifenacin Succinate Tablets should be used with caution in patients being treated for narrow-angle glaucoma
[see Contraindications (4)]. - Who have demonstrated hypersensitivity to solifenacin succinate or the inactive ingredients in Solifenacin Succinate Tablets. Reported adverse reactions have included anaphylaxis and angioedema [see.]
6.2 Postmarketing ExperienceThe following adverse reactions have been identified during post-approval use of solifenacin succinate in the U.S. and/or outside of the U.S. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: peripheral edema, hypersensitivity reactions (including angioedema with airway obstruction, rash, pruritus, urticaria, anaphylactic reaction);Nervous system disorders: dizziness, headache, confusion, hallucinations, delirium, somnolence;Cardiac disorders: QT prolongation, Torsade de Pointes, atrial fibrillation, tachycardia, palpitations;Hepatobiliarydisorders: liver disorders mostly characterized by abnormal liver function tests, AST (aspartate aminotransferase), ALT (alanine aminotransferase), GGT (gamma-glutamyl transferase);Renal and urinary disorders: renal impairment, urinary retention;Metabolism and nutrition disorders: decreased appetite, hyperkalemia;Skin and subcutaneous tissue disorders: exfoliative dermatitis, erythema multiforme, dry skin;Eye disorders:glaucoma;Gastrointestinal disorders:gastroesophageal reflux disease, ileus, vomiting, abdominal pain, dysgeusia, sialadenitis;Respiratory, thoracic and mediastinal disorders: dysphonia, nasal dryness;Musculoskeletal and connective tissue disorders:muscular weakness.
Solifenacin Succinate Tablets are a muscarinic receptor antagonist. Chemically, solifenacin succinate is butanedioic acid, compounded with (1S)-(3R)-1-azabicyclo[2.2.2]oct-3-yl 3,4-dihydro-1-phenyl-2(1H)iso-quinolinecarboxylate (1:1) having an empirical formula of C
23H
26N
2O
2•C
4H
6O
4, and a molecular weight of 480.55. The structural formula of solifenacin succinate is:
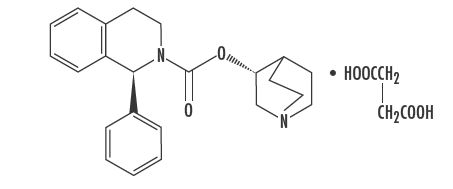
Solifenacin succinate is a white to pale-yellowish-white crystal or crystalline powder. It is freely soluble at room temperature in water, glacial acetic acid, dimethyl sulfoxide, and methanol. Each Solifenacin Succinate tablet contains 5 or 10 mg of solifenacin succinate and is for oral administration. In addition to the active ingredient solifenacin succinate, each Solifenacin Succinate tablet also contains the following inactive ingredients: lactose monohydrate, pregelatinized starch, hypromellose 2910, magnesium stearate, triacetin, polyethylene glycol 3350 and titanium dioxide with yellow ferric oxide and red ferric oxide.
Solifenacin Succinate Tablets were evaluated in four twelve-week, double-blind, randomized, placebo-controlled, parallel group, multicenter clinical trials for the treatment of overactive bladder in adult patients having symptoms of urinary frequency, urgency, and/or urge or mixed incontinence (with a predominance of urge). Entry criteria required that patients have symptoms of overactive bladder for ≥ 3 months duration. These studies involved 3027 patients (1811 on Solifenacin Succinate Tablets and 1216 on placebo), and approximately 90% of these patients completed the 12-week studies. Two of the four studies evaluated the 5 and 10 mg Solifenacin Succinate Tablets doses (Studies 1 and 2) and the other two evaluated only the 10 mg dose (Studies 3 and 4). All patients completing the 12-week studies were eligible to enter an open-label, long-term extension study (Study 5) and 81% of patients enrolling completed the additional 40-week treatment period. The majority of patients were Caucasian (93%) and female (80%) with a mean age of 58 years.
The primary endpoint in all four trials was the mean change from baseline to 12 weeks in number of micturitions/24 hours. Secondary endpoints included mean change from baseline to 12 weeks in number of incontinence episodes/24 hours, and mean volume voided per micturition.
The efficacy of Solifenacin Succinate Tablets was similar across patient age groups and gender. The mean reduction in the number of micturitions per 24 hours was significantly greater with Solifenacin Succinate Tablets 5 mg (2.3; p < 0.001) and Solifenacin Succinate Tablets 10 mg (2.7; p < 0.001) compared to placebo (1.4).The mean reduction in the number of incontinence episodes per 24 hours was significantly greater with Solifenacin Succinate Tablets 5 mg (1.5; p < 0.001) and Solifenacin Succinate Tablets 10 mg (1.8; p < 0.001) treatment groups compared to the placebo treatment group (1.1). The mean increase in the volume voided per micturition was significantly greater with Solifenacin Succinate Tablets 5 mg (32.3 mL; p < 0.001) and Solifenacin Succinate Tablets 10 mg (42.5 mL; p < 0.001) compared with placebo (8.5 mL).
The results for the primary and secondary endpoints in the four individual 12-week clinical studies of Solifenacin Succinate Tablets are reported in Tables 3 through 6.
| Parameter | Placebo (N=253) Mean (SE) | Solifenacin Succinate Tablets 5 mg (N=266) Mean (SE) | Solifenacin Succinate Tablets 10 mg (N=264) Mean (SE) |
|---|---|---|---|
Urinary Frequency (Number of Micturitions/24 hours)* | |||
Baseline Reduction P value vs. placebo | 12.2 (0.26) 1.2 (0.21) | 12.1 (0.24) 2.2 (0.18) < 0.001 | 12.3 (0.24) 2.6 (0.20) < 0.001 |
Number of Incontinence Episodes/24 hours† | |||
Baseline Reduction P value vs. placebo | 2.7 (0.23) 0.8 (0.18) | 2.6 (0.22) 1.4 (0.15) < 0.01 | 2.6 (0.23) 1.5 (0.18) < 0.01 |
Volume Voided per Micturition [mL]† | |||
Baseline Increase P value vs. placebo | 143.8 (3.37) 7.4 (2.28) | 149.6 (3.35) 32.9 (2.92) < 0.001 | 147.2 (3.15) 39.2 (3.11) < 0.001 |
*Primary endpoint | |||
| Parameter | Placebo (N=281) Mean (SE) | Solifenacin Succinate Tablets 5 mg (N=286) Mean (SE) | Solifenacin Succinate Tablets 10 mg (N=290) Mean (SE) |
|---|---|---|---|
Urinary Frequency (Number of Micturitions/24 hours)* | |||
Baseline Reduction P value vs. placebo | 12.3 (0.23) 1.7 (0.19) | 12.1 (0.23) 2.4 (0.17) < 0.001 | 12.1 (0.21) 2.9 (0.18) < 0.001 |
Number of Incontinence Episodes/24 hours† | |||
Baseline Reduction P value vs. placebo | 3.2 (0.24) 1.3 (0.19) | 2.6 (0.18) 1.6 (0.16) < 0.01 | 2.8 (0.20) 1.6 (0.18) 0.016 |
Volume Voided per Micturition [mL]† | |||
Baseline Increase P value vs. placebo | 147.2 (3.18) 11.3 (2.52) | 148.5 (3.16) 31.8 (2.94) < 0.001 | 145.9 (3.42) 36.6 (3.04) < 0.001 |
*Primary endpoint | |||
| Parameter | Placebo (N=309) Mean (SE) | Solifenacin Succinate Tablets 10 mg (N=306) Mean (SE) |
|---|---|---|
Urinary Frequency (Number of Micturitions/24 hours)* | ||
Baseline Reduction P value vs. placebo | 11.5 (0.18) 1.5 (0.15) | 11.7 (0.18) 3.0 (0.15) < 0.001 |
Number of Incontinence Episodes/24 hours† | ||
Baseline Reduction P value vs. placebo | 3.0 (0.20) 1.1 (0.16) | 3.1 (0.22) 2.0 (0.19) < 0.001 |
Volume Voided per Micturition [mL]† | ||
Baseline Increase P value vs. placebo | 190.3 (5.48) 2.7 (3.15) | 183.5 (4.97) 47.2 (3.79) < 0.001 |
*Primary endpoint | ||
| Parameter | Placebo (N=295) Mean (SE) | Solifenacin Succinate Tablets 10 mg (N=298) Mean (SE) |
|---|---|---|
Urinary Frequency (Number of Micturitions/24 hours)* | ||
Baseline Reduction P value vs. placebo | 11.8 (0.18) 1.3 (0.16) | 11.5 (0.18) 2.4 (0.15) < 0.001 |
Number of Incontinence Episodes/24 hours† | ||
Baseline Reduction P value vs. placebo | 2.9 (0.18) 1.2 (0.15) | 2.9 (0.17) 2.0 (0.15) < 0.001 |
Volume Voided per Micturition [mL]† | ||
Baseline Increase P value vs. placebo | 175.7 (4.44) 13.0 (3.45) | 174.1 (4.15) 46.4 (3.73) < 0.001 |
*Primary endpoint | ||
Solifenacin Succinate Tablets are supplied as round, film-coated tablets, biconvex, available in bottles as follows:
Each 5 mg tablet is yellow, debossed with ‘AP28’ on one side and plain on the other side and is available as follows:
Bottle of 30 NDC 35561-285-10
Bottle of 90 NDC 35561-285-11
Bottle of 500 NDC 35561-285-13
Each 10 mg tablet is yellow, debossed with ‘AP29’ on one side and plain on the other side and is available as follows:
Bottle of 30 NDC 35561-286-10
Bottle of 90 NDC 35561-286-11
Bottle of 500 NDC 35561-286-13
Store at 25ºC (77ºF) with excursions permitted from 15ºC to 30ºC (59°F to 86ºF) [see USP Controlled Room Temperature].