Sorbitol
Sorbitol Prescribing Information
3% Sorbitol Urologic Irrigating Solution is indicated for use as a urologic irrigating fluid with endoscopic instruments during transurethral procedures requiring distension, irrigation, and lavage of the urinary bladder. It may be used for lavage of an indwelling catheter to maintain patency.
The volume of solution needed will vary with the nature and duration of the urologic procedure.
If desired, warm in overwrap to near body temperature in a water bath or oven heated to not more than 45°C.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Anuria.
The literature reports occasional adverse reactions for intravenous sorbitol infusions. These include disturbances such as acidosis, electrolyte loss, marked diuresis, urinary retention, edema, dryness of mouth and thirst, and dehydration; cardiovascular/pulmonary disorders such as pulmonary congestion, hypotension, tachycardia, angina-like pains, and other general reactions such as blurred vision, convulsions, nausea, vomiting, diarrhea, rhinitis, chills, vertigo, and backache. Allergic reactions reported to occur from sorbitol include urticaria.
Should adverse reactions occur, discontinue the irrigant and reevaluate the clinical status of the patient.
3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution in a single dose UROMATIC Plastic Container for use as a urologic irrigating solution. Each liter contains 30 g Sorbitol in Water for Injection. pH 5.0 (4.5 to 6.5). Osmolarity 165 mOsmol/L (calc.). No antimicrobial agent has been added.
Sorbitol is a reduced form of dextrose and is designated chemically as D-Glucitol,
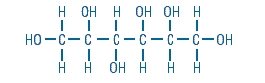
The UROMATIC plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million; however, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
3% Sorbitol Urologic Irrigating Solution is useful as an irrigating fluid for the urinary bladder because the sorbitol solution is nonhemolytic, electrically nonconductive, and provides a high degree of visibility for urologic procedures requiring endoscopy. During transurethral surgical procedures, the solution acts as a lavage for removing blood and tissue fragments. It is also useful as an irrigating fluid to maintain the patency of an indwelling catheter in the immediate postoperative period. If absorbed either intravascularly or extravascularly during transurethral resections, the sorbitol will be either metabolized to carbon dioxide and water via the fructose pathway, or excreted by a normally functioning kidney.