Sotalol Hydrochloride Prescribing Information
Sotalol hydrochloride tablets (AF) are indicated for the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are currently in sinus rhythm. Because sotalol hydrochloride tablets (AF) can cause life-threatening ventricular arrhythmias, it should be reserved for patients in whom AFIB/AFL is highly symptomatic. Patients with paroxysmal AFIB whose AFIB/AFL that is easily reversed (by Valsalva maneuver, for example) should usually not be given sotalol hydrochloride tablets (AF) (see
In eight controlled trials of patients with AFIB/AFL and other supraventricular arrhythmias (N=659) there were four cases of Torsade de Pointes reported (0.6%) during the controlled phase of treatment with sotalol (AF). The incidence of Torsade de Pointes was significantly lower in those patients receiving total daily doses of 320 mg or less (0.3%), as summarized in Table 5 below. Both patients who had Torsade de Pointes in the group receiving >320 mg/day were receiving 640 mg/day. In the group receiving ≤320 mg daily, one case of TdP occurred at a daily dose of 320 mg on day 4 of treatment and one case occurred on a daily dose of 160 mg on day 1 of treatment.
Sotalol (AF) (Daily Dose) | |||||
Any Dose (N=659) | >320 mg/day (N=62) | ≤320 mg/day (N=597) | ≤240 mg/day (N=340) | Placebo (N=358) | |
n (%) | n (%) | n (%) | n (%) | n (%) | |
Torsade de Pointes | 4(0.6%) | 2(3.2%) | 2(0.3%) | 1(0.3%) | 0 |
Prolongation of the QT interval is dose related, increasing from baseline an average of 25, 40, and 50 msec in the 80, 120, and 160 mg groups, respectively, in the clinical dose-response study. In this clinical trial sotalol (AF) treatment was not initiated if the QT interval was greater than 450 msec and during therapy the dose was reduced or discontinued if the QT interval was ≥520 msec.
Experience in patients with ventricular arrhythmias is also pertinent to the risk of Torsade de Pointes in patients with AFIB/AFL (see below).
In patients with a history of sustained ventricular tachycardia, the incidence of Torsade de Pointes during sotalol treatment was 4% and worsened VT in about 1%; in patients with other less serious ventricular arrhythmias the incidence of Torsade de Pointes was 1% and new or worsened VT in about 0.7%. Additionally, in approximately 1% of patients, deaths were considered possibly drug related; such cases, although difficult to evaluate, may have been associated with proarrhythmic events.
Torsade de Pointes arrhythmias in patients with VT/VF were dose related, as was the prolongation of QT (QTc) interval, as shown in Table 6 below.
Daily Dose (mg) | Incidence of Torsade de Pointes | Mean QTc*(msec) |
80 | 0 (69) † | 463 (17) † |
160 | 0.5 (832) † | 467 (181) † |
320 | 1.6 (835) † | 473 (344) † |
480 | 4.4 (459) † | 483 (234) † |
640 | 3.7 (324) † | 490 (185) † |
>640 | 5.8 (103) † | 512 (62) † |
*highest on therapy value †( ) Number of patients assessed | ||
Table 7 below relates the incidence of Torsade de Pointes to on-therapy QTcand change in QTcfrom baseline. It should be noted, however, that the highest on therapy QTcwas in many cases the one obtained at the time of the Torsade de Pointes event, so that the table overstates the predictive value of a high QTc.
Table 7 Relationship Between QTcInterval Prolongation and Torsade de Pointe
On-Therapy QTc Interval (msec) | Incidence of Torsade de Pointes | Change in QTc Interval From Baseline (msec) | Incidence of Torsade de Pointes |
Less than 500 | 1.3% (1787)* | Less than 65 | 1.6% (1516)* |
500-525 | 3.4% (236)* | 65-80 | 3.2% (158)* |
525-550 | 5.6 % (125)* | 80-100 | 4.1% (146)* |
>550 | 10.8% (157)* | 100-130 | 5.2% (115)* |
>130 | 7.1% (99)* | ||
*( ) Number of patients assessed | |||
In addition to dose and presence of sustained VT, other risk factors for Torsade de Pointes were gender (females had a higher incidence), excessive prolongation of the QTcinterval and history of cardiomegaly or congestive heart failure. Patients with sustained ventricular tachycardia and a history of congestive heart failure appear to have the highest risk for serious proarrhythmia (7%).
Of the ventricular arrhythmia patients experiencing Torsade de Pointes, approximately two-thirds spontaneously reverted to their baseline rhythm. The others were either converted electrically (D/C cardioversion or overdrive pacing) or treated with other drugs (see
The use of sotalol (AF) in conjunction with other drugs that prolong the QT interval has not been studied and is not recommended. Such drugs include many antiarrhythmics, some phenothiazines, bepridil, tricyclic antidepressants, and certain oral macrolides. Class I or Class III antiarrhythmic agents should be withheld for at least three half-lives prior to dosing with sotalol (AF). In clinical trials, sotalol (AF) was not administered to patients previously treated with oral amiodarone for >1 month in the previous three months.
Class Ia antiarrhythmic drugs, such as disopyramide, quinidine and procainamide and other Class III drugs (e.g., amiodarone) are not recommended as concomitant therapy with sotalol (AF), because of their potential to prolong refractoriness (see
Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta-blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure. In patients who have heart failure controlled by digitalis and/or diuretics, sotalol hydrochloride tablets (AF) should be administered cautiously. Both digitalis and sotalol slow AV conduction. As with all beta-blockers, caution is advised when initiating therapy in patients with any evidence of left ventricular dysfunction. In a pooled data base of four placebo-controlled AFIB/AFL and PSVT studies, new or worsening CHF occurred during therapy with sotalol (AF) in 5 (1.2%) of 415 patients. In these studies patients with uncontrolled heart failure were excluded (i.e.,NYHA Functional Classes III or IV). In other premarketing sotalol studies, new or worsened congestive heart failure (CHF) occurred in 3.3% (n=3257) of patients and led to discontinuation in approximately 1% of patients receiving sotalol. The incidence was higher in patients presenting with sustained ventricular tachycardia/fibrillation (4.6%, n=1363), or a prior history of heart failure (7.3%, n=696). Based on a life-table analysis, the one-year incidence of new or worsened CHF was 3% in patients without a prior history and 10% in patients with a prior history of CHF. NYHA Classification was also closely associated to the incidence of new or worsened heart failure while receiving sotalol (1.8% in 1395 Class I patients, 4.9% in 1254 Class II patients and 6.1% in 278 Class III or IV patients).
Sotalol (AF) should not be used in patients with hypokalemia or hypomagnesemia prior to correction of imbalance, as these conditions can exaggerate the degree of QT prolongation, and increase the potential for Torsade de Pointes. Special attention should be given to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or patients receiving concomitant diuretic drugs.
The incidence of bradycardia (as determined by the investigators) in the supraventricular arrhythmia population treated with sotalol (AF) (N = 415) was 13%, and led to discontinuation in 2.4% of patients. Bradycardia itself increases the risk of Torsade de Pointes.
Sotalol has been used in a controlled trial following an acute myocardial infarction without evidence of increased mortality (see
Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy. Occasional cases of exacerbation of angina pectoris, arrhythmias and, in some cases, myocardial infarction have been reported after abrupt discontinuation of beta-blocker therapy. Therefore, it is prudent when discontinuing chronically administered sotalol (AF), particularly in patients with ischemic heart disease, to carefully monitor the patient and consider the temporary use of an alternate beta-blocker if appropriate. If possible, the dosage of sotalol (AF) should be gradually reduced over a period of one to two weeks. If angina or acute coronary insufficiency develops, appropriate therapy should be instituted promptly. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized in patients receiving sotalol (AF), abrupt discontinuation in patients with arrhythmias may unmask latent coronary insufficiency.
While taking beta-blockers, patients with a history of anaphylactic reaction to a variety of allergens may have a more severe reaction on repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat the allergic reaction.
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
In patients with diabetes (especially labile diabetes) or with a history of episodes of spontaneous hypoglycemia, sotalol (AF) should be given with caution since beta-blockade may mask some important premonitory signs of acute hypoglycemia; e.g., tachycardia.
Sotalol (AF) should be used only with extreme caution in patients with sick sinus syndrome associated with symptomatic arrhythmias, because it may cause sinus bradycardia, sinus pauses or sinus arrest. In patients with AFIB and sinus node dysfunction, the risk of Torsade de Pointes with sotalol (AF) therapy is increased, especially after cardioversion. Bradycardia following cardioversion in these patients is associated with QTcinterval prolongation which is augmented due to the reverse use dependence of the Class III effects of sotalol (AF). Patients with AFIB/AFL associated with the sick sinus syndrome may be treated with sotalol (AF) if they have an implanted pacemaker for control of bradycardia symptoms.
Beta-blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-blockade which might be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm. The beta-blocking effects of sotalol hydrochloride tablets may be useful in controlling heart rate in AFIB associated with thyrotoxicosis but no study has been conducted to evaluate this.
In general, antiarrhythmic therapy for AFIB/AFL aims to prolong the time in normal sinus rhythm. Recurrence is expected in some patients (see
Prolongation of Time to Recurrence of Symptomatic Atrial Fibrillation/ Flutter
Sotalol hydrochloride has been studied in patients with symptomatic AFIB/AFL in two principal studies, one in patients with primarily paroxysmal AFIB/AFL, the other in patients with primarily chronic AFIB.
In one study, a U.S. multicenter, randomized, placebo-controlled, double-blind, dose-response trial of patients with symptomatic primarily paroxysmal AFIB/AFL, three fixed dose levels of sotalol hydrochloride (80 mg, 120 mg and 160 mg) twice daily and placebo were compared in 253 patients. In patients with reduced creatinine clearance (40-60 mL/min) the same doses were given once daily. Patients were not randomized for the following reasons: QT >450 msec; creatinine clearance <40 mL/min; intolerance to beta-blockers; bradycardia-tachycardia syndrome in the absence of an implanted pacemaker; AFIB/AFL was asymptomatic or was associated with syncope, embolic CVA or TIA; acute myocardial infarction within the previous 2 months; congestive heart failure; bronchial asthma or other contraindications to beta-blocker therapy; receiving potassium losing diuretics without potassium replacement or without concurrent use of ACE-inhibitors; uncorrected hypokalemia (serum potassium <3.5 meq/L) or hypomagnesemia (serum magnesium <1.5 meq/L); received chronic oral amiodarone therapy for >1 month within previous 12 weeks; congenital or acquired long QT syndromes; history of Torsade de Pointes with other antiarrhythmic agents which increase the duration of ventricular repolarization; sinus rate <50 bpm during waking hours; unstable angina pectoris; receiving treatment with other drugs that prolong the QT interval; and AFIB/AFL associated with the Wolff-Parkinson-White (WPW) syndrome. If the QT interval increased to ≥520 msec (or JT ≥430 msec if QRS >100 msec) the drug was discontinued. The patient population in this trial was 64% male, and the mean age was 62 years. No structural heart disease was present in 43% of the patients. Doses were administered once daily in 20% of the patients because of reduced creatinine clearance.
Sotalol hydrochloride was shown to prolong the time to the first symptomatic, ECG-documented recurrence of AFIB/AFL, as well as to reduce the risk of such recurrence at both 6 and 12 months. The 120 mg dose was more effective than 80 mg, but 160 mg did not appear to have an added benefit. Note that these doses were given twice or once daily, depending on renal function. The results are shown in Figure 1 and Tables 1 and 2.
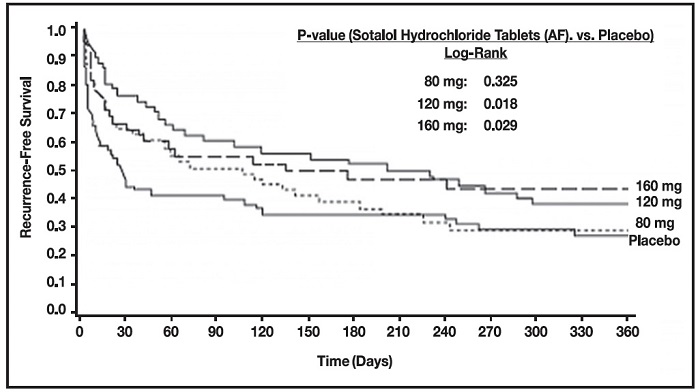
Figure 1 Study 1—Time to First ECG-Documented Recurrence of Symptomatic AFIB/AFL Since Randomization
Table 1 Study 1 – Patient Status at 12 Months
Placebo | Sotalol Hydrochloride (AF) Dose | |||
80 mg | 120 mg | 160 mg | ||
Randomized | 69 | 59 | 63 | 62 |
On treatment in NSR at 12 months without recurrence* | 23% | 22% | 29% | 23% |
Recurrence*† | 67% | 58% | 49% | 42% |
D/C for AEs | 6% | 12% | 18% | 29% |
*Symptomatic AFIB/AFL †Efficacy endpoint of Study 1; study treatment stopped. | ||||
Please note that columns do not add up to 100% due to discontinuations (D/C) for “other” reasons.
Table 2 Study 1 – Median Time to Recurrence of Symptomatic AFIB/AFL and Relative Risk (vs. Placebo) at 12 Months
Placebo | Sotalol Hydrochloride Dose | |||
80 mg | 120 mg | 160 mg | ||
p-value vs placebo | P=0.325 | P=0.018 | P=0.029 | |
Relative Risk (RR) to placebo | 0.81 | 0.59 | 0.59 | |
Median time to recurrence (days) | 27 | 106 | 229 | 175 |
Discontinuation because of adverse events was dose related.
In a second multicenter, randomized, placebo-controlled, double-blind study of 6 months duration in 232 patients with chronic AFIB, sotalol hydrochloride (AF) was titrated over a dose range from 80 mg/day to 320 mg/day. The patient population of this trial was 70% male with a mean age of 65 years. Structural heart disease was present in 49% of the patients. All patients had chronic AFIB for >2 weeks but <1 year at entry with a mean duration of 4.1 months. Patients were excluded if they had significant electrolyte imbalance, QTc>460 msec, QRS >140 msec, any degree of AV block or functioning pacemaker, uncompensated cardiac failure, asthma, significant renal disease (estimated creatinine clearance <50 mL/min), heart rate <50 bpm, myocardial infarction or open heart surgery in past 2 months, unstable angina, infective endocarditis, active pericarditis or myocarditis, ≥3 DC cardioversions in the past, medications that prolonged QT interval, and previous amiodarone treatment. After successful cardioversion patients were randomized to receive placebo (n=114) or sotalol hydrochloride (AF) (n=118), at a starting dose of 80 mg twice daily. If the initial dose was not tolerated it was decreased to 80 mg once daily, but if it was tolerated it was increased to 160 mg twice daily. During the maintenance period 67% of treated patients received a dose of 160 mg twice daily, and the remainder received doses of 80 mg once daily (17%) and 80 mg twice daily (16%).
Figure 2 and Tables 3 and 4 show the results of the trial. There was a longer time to ECG-documented recurrence of AFIB and a reduced risk of recurrence at 6 months compared to placebo.
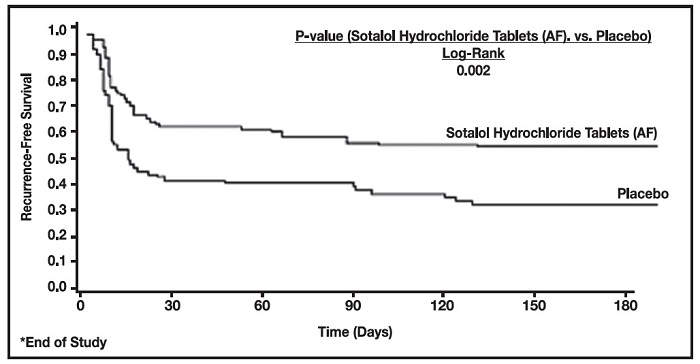
Figure 2 Study 2 – Time to First ECG-Documented Recurrence of Symptomatic AFIB/AFL/Death Since Randomization
Sotalol Hydrochloride (AF) | Placebo | |
Randomized | 118 | 114 |
On treatment in NSR at 6 months without recurrence* | 45% | 29% |
Recurrence*† | 49% | 67% |
D/C for AEs | 6% | 3% |
Death | 1% | |
*Symptomatic or asymptomatic AFIB/AFL †Efficacy endpoint of Study 2; study treatment stopped. | ||
Sotalol Hydrochloride | Placebo | |
p-value vs placebo | p=0.002 | |
Relative Risk (RR) to placebo | 0.55 | |
Median time to recurrence (days) | >180 | 44 |
In a multicenter double-blind randomized study reported by D. Julian et al, the effect of sotalol 320 mg once daily was compared with that of placebo in 1456 patients (randomized 3:2, sotalol to placebo) surviving an acute myocardial infarction (MI). Treatment was started 5-14 days after infarction. Patients were followed for 12 months. The mortality rate was 7.3% in the sotalol group and 8.9% in the placebo group, not a statistically significant difference. Although the results do not show evidence of a benefit of sotalol in this population, they do not show an added risk in post MI patients receiving sotalol.


Sotalol hydrochloride tablets (AF) are also indicated for the treatment of documented life-threatening ventricular arrhythmias and is marketed under the brand name
• Therapy with sotalol (AF) must be initiated (and, if necessary, titrated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Patients should continue to be monitored in this way for a minimum of 3 days on the maintenance dose. In addition, patients should not be discharged within 12 hours of electrical or pharmacological conversion to normal sinus rhythm.
• The QT interval is used to determine patient eligibility for sotalol hydrochloride tablet (AF) treatment and for monitoring safety during treatment. The baseline QT interval must be ≤450 msec in order for a patient to be started on sotalol hydrochloride tablet (AF) therapy. During initiation and titration, the QT interval should be monitored 2-4 hours after each dose. If the QT interval prolongs to 500 msec or greater, the dose must be reduced or the drug discontinued.
•
If the 80 mg dose level (given BID or QD depending upon the creatinine clearance) does not reduce the frequency of relapses of AFIB/AFL and is tolerated without excessive QT interval prolongation (i.e., ≥520 msec), the dose level may be increased to 120 mg (BID or QD depending upon the creatinine clearance). As proarrhythmic events can occur not only at initiation of therapy, but also with each upward dosage adjustment, Steps 2 through 5 used during initiation of sotalol hydrochloride tablets (AF) therapy should be followed when increasing the dose level. In the U.S. multicenter dose-response study, the 120 mg dose (BID or QD) was found to be the most effective in prolonging the time to ECG documented symptomatic recurrence of AFIB/AFL. If the 120 mg dose does not reduce the frequency of early relapse of AFIB/AFL and is tolerated without excessive QT interval prolongation (≥520 msec), an increase to 160 mg (BID or QD depending upon the creatinine clearance), can be considered. Steps 2 through 5 used during the initiation of therapy should be used again to introduce such an increase.
• Patients with atrial fibrillation should be anticoagulated according to usual medical practice. Hypokalemia should be corrected before initiation of sotalol hydrochloride tablet (AF) therapy (see
• Patients to be discharged on sotalol hydrochloride tablets (AF) therapy from an in-patient setting should have an adequate supply of sotalol hydrochloride tablets (AF), to allow uninterrupted therapy until the patient can fill a sotalol hydrochloride tablets (AF) prescription.
Sotalol hydrochloride tablets (AF) are contraindicated in patients with sinus bradycardia (<50 bpm during waking hours), sick sinus syndrome or second and third degree AV block (unless a functioning pacemaker is present), congenital or acquired long QT syndromes, baseline QT interval >450 msec, cardiogenic shock, uncontrolled heart failure, hypokalemia (<4 meq/L), creatinine clearance <40 mL/min, bronchial asthma and previous evidence of hypersensitivity to sotalol.
Adverse events that are clearly related to sotalol (AF) are those which are typical of its Class II (beta-blocking) and Class III (cardiac action potential duration prolongation) effects. The common documented beta-blocking adverse events (bradycardia, dyspnea, and fatigue) and Class III effects (QT interval prolongation) are dose related.
In a pooled clinical trial population consisting of four placebo-controlled studies with 275 patients with AFIB/AFL treated with 160-320 mg doses of sotalol (AF), the following adverse events were reported at a rate of 2% or more in the 160-240 mg treated patients and greater than the rate in placebo patients (See Table 8). The data are presented by incidence of events in the sotalol (AF) and placebo groups by body system and daily dose. No significant irreversible non-cardiac end-organ toxicity was observed.
Table 8 Incidence (%) of Common Adverse Events (≥2% in the 160-240 mg group and more frequent than on placebo) in Four Placebo-Controlled Studies of Patients with AFIB/AFL
Placebo | Sotolol (AF) Total Daily Dose | ||
Body System/Adverse Event (Preferred Term) | N=282 | 160-240 N=153 | >240-320 N=122 |
CARDIOVASCULAR | |||
Abnormality ECG | 0.4 | 3.3 | 2.5 |
Angina Pectoris | 1.1 | 2.0 | 1.6 |
Bradycardia | 2.5 | 13.1 | 12.3 |
Chest Pain Cardiac/Non-Anginal | 4.6 | 4.6 | 2.5 |
Disturbance Rhythm Atrial | 2.1 | 2.0 | 1.6 |
Disturbance Rhythm Subjective | 9.9 | 9.8 | 7.4 |
GASTROINTESTINAL | |||
Appetite Decreased | 0.4 | 2.0 | 1.6 |
Diarrhea | 2.1 | 5.2 | 5.7 |
Distention Abdomen | 0.4 | 0.7 | 2.5 |
Dyspepsia/Heartburn | 1.8 | 2.0 | 2.5 |
Nausea/Vomiting | 5.3 | 7.8 | 5.7 |
Pain Abdomen | 2.5 | 3.9 | 2.5 |
GENERAL | |||
Fatigue | 8.5 | 19.6 | 18.9 |
Fever | 0.7 | 0.7 | 3.3 |
Hyperhidrosis | 3.2 | 5.2 | 4.9 |
Influenza | 0.4 | 2.0 | 0.8 |
Sensation Cold | 0.7 | 2.0 | 2.5 |
Weakness | 3.2 | 5.2 | 4.9 |
MUSCULOSKELETAL/CONNECTIVE TISSUE | |||
Pain Chest Musculoskeletal | 1.4 | 2.0 | 2.5 |
Pain Musculoskeletal | 2.8 | 2.6 | 4.1 |
NERVOUS SYSTEM | |||
Dizziness | 12.4 | 16.3 | 13.1 |
Headache | 5.3 | 3.3 | 11.5 |
Insomnia | 1.1 | 2.6 | 4.1 |
RESPIRATORY | |||
Cough | 2.5 | 3.3 | 2.5 |
Dyspnea | 7.4 | 9.2 | 9.8 |
Infection Upper Respiratory | 1.1 | 2.6 | 3.3 |
Tracheobronchitis | 0.7 | 0.7 | 3.3 |
SPECIAL SENSES | |||
Disturbance Vision | 0.7 | 2.6 | 0.8 |
Overall, discontinuation because of unacceptable adverse events was necessary in 17% of the patients, and occurred in 10% of patients less than two weeks after starting treatment. The most common adverse events leading to discontinuation of sotalol (AF) were: fatigue 4.6%, bradycardia 2.4%, proarrhythmia 2.2%, dyspnea 2%, and QT interval prolongation 1.4%.
In clinical trials involving 1292 patients with sustained VT/VF, the common adverse events (occurring in ≥2% of patients) were similar to those described for the AFIB/AFL population.
Occasional reports of elevated serum liver enzymes have occurred with sotalol therapy but no cause and effect relationship has been established. One case of peripheral neuropathy which resolved on discontinuation of sotalol and recurred when the patient was rechallenged with the drug was reported in an early dose tolerance study. Elevated blood glucose levels and increased insulin requirements can occur in diabetic patients.
In an unblinded multicenter trial of 25 patients with SVT and/or VT receiving daily doses of 30, 90 and 210 mg/m 2 with dosing every 8 hours for a total of 9 doses, no Torsades de Pointes or other serious new arrhythmias were observed. One (1) patient, receiving 30 mg/m 2 daily, was discontinued because of increased frequency of sinus pauses/bradycardia. Additional cardiovascular AEs were seen at the 90 and 210 mg/m 2 daily dose levels. They included QT prolongations (2 patients), sinus pauses/bradycardia (1 patient), increased severity of atrial flutter and reported chest pain (1 patient). Values for QT c ≥525 msec were seen in 2 patients at the 210 mg/m 2 daily dose level. Serious adverse events including death, Torsades de Pointes, other proarrhythmias, high-degree A-V blocks and bradycardia have been reported in infants and/or children.
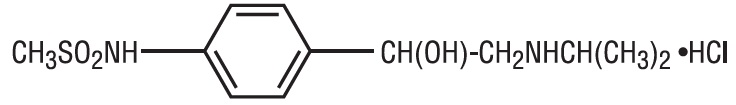
Sotalol hydrochloride tablets, USP (AF) are an antiarrhythmic drug with Class II (beta-adrenoreceptor blocking) and Class III (cardiac action potential duration prolongation) properties. It is supplied as a white, capsule-shaped tablet for oral administration. Sotalol hydrochloride is a white, crystalline solid with a molecular weight of 308.8. It is hydrophilic, soluble in water, propylene glycol and ethanol, but is only slightly soluble in chloroform. Chemically, sotalol hydrochloride is d, l-
Sotalol hydrochloride tablets, USP (AF) contain the following inactive ingredients: pregelatinized starch, microcrystalline cellulose, lactose monohydrate, colloidal silicon dioxide, stearic acid, and magnesium stearate.