Sumatriptan Succinate
Sumatriptan Succinate Prescribing Information
Sumatriptan tablets are indicated for the acute treatment of migraine with or without aura in adults.
• Use only if a clear diagnosis of migraine headache has been established. If a patient has no response to the first migraine attack treated with sumatriptan, reconsider the diagnosis of migraine before sumatriptan tablets are administered to treat any subsequent attacks.
• Sumatriptan tablets are not indicated for the prevention of migraine attacks.
• Safety and effectiveness of sumatriptan tablets have not been established for cluster headache.
50 m g Tablets: White, round, biconvex film-coated tablets debossed with “RDY” on one side and “292” on the other side.
100 mg Tablets: White, capsule shaped, biconvex film-coated tablets debossed with “RDY” on one side and “293” on the other side.
Sumatriptan tablets are contraindicated in patients with:
• Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (
The use of sumatriptan tablets are contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan tablets. Some of these reactions occurred in patients without known CAD. Sumatriptan tablets may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets. If there is evidence of CAD or coronary artery vasospasm, sumatriptan tablets are contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of sumatriptan tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of sumatriptan tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of sumatriptan tablets.
• Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1agonists. Discontinue sumatriptan tablets if these disturbances occur. Sumatriptan tablets are contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
• History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue sumatriptan tablets if a cerebrovascular event occurs.
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan tablets are contraindicated in patients with a history of stroke or TIA.
• Peripheral vascular disease [see Warnings and Precautions (
Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1agonists have not been clearly established.
• Ischemic bowel disease [see Warnings and Precautions (
Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1agonists have not been clearly established.
• Uncontrolled hypertension [see Warnings and Precautions (
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan tablets are contraindicated in patients with uncontrolled hypertension.
• Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine1 (5-HT1) agonist [see Drug Interactions (
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and sumatriptan tablets within 24 hours of each other is contraindicated.
Because their vasospastic effects may be additive, co
administration of sumatriptan tablets and other 5-HT1agonists (e.g., triptans) within 24 hours of each other is contraindicated.• Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (
MAO-A inhibitors increase systemic exposure by 7-fold. Therefore, the use of sumatriptan tablets in patients receiving MAO-A inhibitors is contraindicated [see Clinical Pharmacology (
The mean maximum concentration following oral dosing with 25 mg is 18 ng/mL (range: 7 to 47 ng/mL) and 51 ng/mL (range: 28 to 100 ng/mL) following oral dosing with 100 mg of sumatriptan. This compares with a Cmaxof 5 and 16 ng/mL following dosing with a 5 and 20 mg intranasal dose, respectively. The mean Cmaxfollowing a 6-mg subcutaneous injection is 71 ng/mL (range: 49 to 110 ng/mL). The bioavailability is approximately 15%, primarily due to presystemic metabolism and partly due to incomplete
absorption. The Cmaxis similar during a migraine attack and during a migraine-free period, but the Tmaxis slightly later during the attack, approximately 2.5 hours compared with 2 hours. When given as a single dose, sumatriptan displays dose proportionality in its extent of absorption (area under the curve [AUC]) over the dose range of 25 to 200 mg, but the Cmaxafter 100 mg is approximately 25% less than expected (based on the 25 mg dose).Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The apparent volume of distribution is 2.7 L/kg.
In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
The elimination half-life of sumatriptan is approximately 2.5 hours. Radiolabeled14C-sumatriptan administered orally is largely renally excreted (about 60%) with about 40% found in the feces. Most of the radiolabeled compound excreted in the urine is the major metabolite, IAA, which is inactive, or the IAA glucuronide. Only 3% of the dose can be recovered as unchanged sumatriptan.
The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied. The use of sumatriptan tablets in this population is contraindicated [see Contraindications (
Due to gut and hepatic metabolic first-pass effects, the increase of systemic exposure after coadministration of an MAO-A inhibitor with oral sumatriptan is greater than after coadministration of the MAO inhibitors with subcutaneous sumatriptan.
In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
A small trial evaluating the effect of pretreatment with an MAO-A inhibitor on the bioavailability from a 25-mg oral sumatriptan tablet resulted in an approximately 7-fold increase in systemic exposure.
Alcohol: Alcohol consumed 30 minutes prior to sumatriptan ingestion had no effect on the pharmacokinetics of sumatriptan.
• Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen) [see Warnings and Precautions (
Anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan tablets are contraindicated in patients with a history of hypersensitivity reaction to sumatriptan.
• Severe hepatic impairment [see Use in Specific Populations (
The maximum single dose in patients with mild to moderate hepatic impairment should not exceed 50 mg. Sumatriptan tablets are contraindicated in patients with severe hepatic impairment [see Clinical Pharmacology (
The mean maximum concentration following oral dosing with 25 mg is 18 ng/mL (range: 7 to 47 ng/mL) and 51 ng/mL (range: 28 to 100 ng/mL) following oral dosing with 100 mg of sumatriptan. This compares with a Cmaxof 5 and 16 ng/mL following dosing with a 5 and 20 mg intranasal dose, respectively. The mean Cmaxfollowing a 6-mg subcutaneous injection is 71 ng/mL (range: 49 to 110 ng/mL). The bioavailability is approximately 15%, primarily due to presystemic metabolism and partly due to incomplete
absorption. The Cmaxis similar during a migraine attack and during a migraine-free period, but the Tmaxis slightly later during the attack, approximately 2.5 hours compared with 2 hours. When given as a single dose, sumatriptan displays dose proportionality in its extent of absorption (area under the curve [AUC]) over the dose range of 25 to 200 mg, but the Cmaxafter 100 mg is approximately 25% less than expected (based on the 25 mg dose).Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The apparent volume of distribution is 2.7 L/kg.
In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
The elimination half-life of sumatriptan is approximately 2.5 hours. Radiolabeled14C-sumatriptan administered orally is largely renally excreted (about 60%) with about 40% found in the feces. Most of the radiolabeled compound excreted in the urine is the major metabolite, IAA, which is inactive, or the IAA glucuronide. Only 3% of the dose can be recovered as unchanged sumatriptan.
The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied. The use of sumatriptan tablets in this population is contraindicated [see Contraindications (
Due to gut and hepatic metabolic first-pass effects, the increase of systemic exposure after coadministration of an MAO-A inhibitor with oral sumatriptan is greater than after coadministration of the MAO inhibitors with subcutaneous sumatriptan.
In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
A small trial evaluating the effect of pretreatment with an MAO-A inhibitor on the bioavailability from a 25-mg oral sumatriptan tablet resulted in an approximately 7-fold increase in systemic exposure.
Alcohol: Alcohol consumed 30 minutes prior to sumatriptan ingestion had no effect on the pharmacokinetics of sumatriptan.
The following adverse reactions are discussed in more detail in other sections of the prescribing information:
• Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see
The use of sumatriptan tablets are contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of sumatriptan tablets. Some of these reactions occurred in patients without known CAD. Sumatriptan tablets may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving sumatriptan tablets. If there is evidence of CAD or coronary artery vasospasm, sumatriptan tablets are contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of sumatriptan tablets in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of sumatriptan tablets. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of sumatriptan tablets.
• Arrhythmias [see
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1agonists. Discontinue sumatriptan tablets if these disturbances occur. Sumatriptan tablets are contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
• Chest, throat, neck, and/or jaw pain/tightness/pressure [see
Sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with sumatriptan tablets and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of sumatriptan tablets are contraindicated in patients with CAD and those with Prinzmetal’s variant angina.
• Cerebrovascular events [see
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue sumatriptan tablets if a cerebrovascular event occurs.
Before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, exclude other potentially serious neurological conditions. Sumatriptan tablets are contraindicated in patients with a history of stroke or TIA.
• Other vasospasm reactions [see
Sumatriptan tablets may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1agonist, rule out a vasospastic reaction before receiving additional sumatriptan tablets.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1agonists have not been clearly established.
• Medication overuse headache [see
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
• Serotonin syndrome [see
Serotonin syndrome may occur with sumatriptan tablets, particularly during coadministration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs),
• Increase in blood pressure [see
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with sumatriptan. Sumatriptan tablets are contraindicated in patients with uncontrolled hypertension.
• Hypersensitivity reactions [see
Sumatriptan tablets are contraindicated in patients with:
• Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (
• Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (
• History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (
• Peripheral vascular disease [see Warnings and Precautions (
• Ischemic bowel disease [see Warnings and Precautions (
• Uncontrolled hypertension [see Warnings and Precautions (
• Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine1 (5-HT1) agonist [see Drug Interactions (
• Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (
• Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen) [see Warnings and Precautions (
• Severe hepatic impairment [see Use in Specific Populations (
- History of coronary artery disease or coronary artery vasospasm (4)
- Wolff-Parkinson-White syndrome or other cardiac accessory conduction pathway disorders (4)
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine (4)
- Peripheral vascular disease (4)
- Ischemic bowel disease (4)
- Uncontrolled hypertension (4)
- Recent (within 24 hours) use of another 5-HT1agonist (e.g., another triptan) or of an ergotamine-containing medication. (4)
- Concurrent or recent (past 2 weeks) use of monoamine oxidase-A inhibitor. (4)
- Hypersensitivity to sumatriptan (angioedema and anaphylaxis seen). (4)
- Severe hepatic impairment. (4)
Anaphylactic/anaphylactoid reactions have occurred in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. Sumatriptan tablets are contraindicated in patients with a history of hypersensitivity reaction to sumatriptan.
• Seizures [see
Seizures have been reported following administration of sumatriptan. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. Sumatriptan tablets should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
Sumatriptan tablets USP contain sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
The molecular formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate USP is a white or almost white powder that is freely soluble in water, sparingly soluble in methanol, practically inso
luble in methylene chloride. Each sumatriptan tablet USP for oral administration contains 35, 70, or 140 mg of sumatriptan succinate USP equivalent to 25, 50, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients croscarmellose sodium, lactose anhydrous, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, talc, titanium dioxide and triacetin.
The efficacy of sumatriptan tablets in the acute treatment of migraine headaches was demonstrated in 3, randomized, double-blind, placebo-controlled trials. Patients enrolled in these 3 trials were predominately female (87%) and Caucasian (97%), with a mean age of 40 years (range: 18 to 65 years). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in
In all 3 trials, the percentage of patients achieving headache response 2 and 4 hours after treatment was significantly greater among patients receiving sumatriptan tablets at all doses compared with those who received placebo. In 1 of the 3 trials, there was a statistically significant greater percentage of patients with headache response at 2 and 4 hours in the 50-mg or 100-mg group when compared with the 25-mg dose groups. There were no statistically significant differences between the 50-mg and 100-mg dose groups in any trial. The results from the 3 controlled clinical trials are summarized in Table 2.
Sumatriptan Tablets 25 mg 2 hr 4 hr | Sumatriptan Tablets 50 mg 2 hr 4 hr | Sumatriptan Tablets 100 mg 2 hr 4 hr | Placebo 2 hr 4 hr | |
| Trial 1 | 52%a 67%a (n = 298) | 61%a,b 78%a,b (n = 296) | 62%a,b 79%a,b (n = 296) | 27% 38% (n = 94) |
| Trial 2 | 52%a 70%a (n = 66) | 50%a 68%a (n = 62) | 56%a 71%a (n = 66) | 26% 38% (n = 65) |
| Trial 3 | 52%a 65%a (n = 48) | 54%a 72%a (n = 46) | 57%a 78%a (n = 46) | 17% 19% (n = 47) |
a P<0.05 in comparison with placebo.
b P<0.05 in comparison with 25 mg.
The estimated probability of achieving an initial headache response over the 4 hours following treatment in pooled Trials 1, 2, and 3 is depicted in Figure 1.
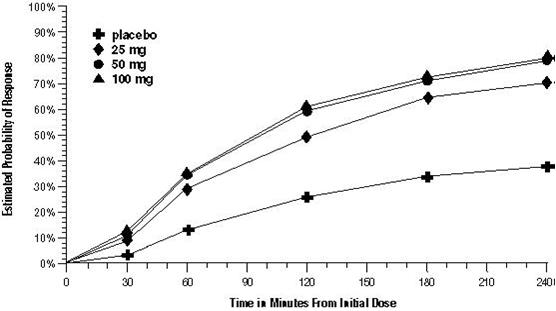
For patients with migraine-associated nausea, photophobia, and/or phonophobia at baseline, there was a lower incidence of these symptoms at 2 hours (Trial 1) and at 4 hours (Trials 1, 2, and 3) following administration of sumatriptan tablets compared with placebo.
As early as 2 hours in Trials 2 and 3, or as early as 4 hours in Trial 1, through 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
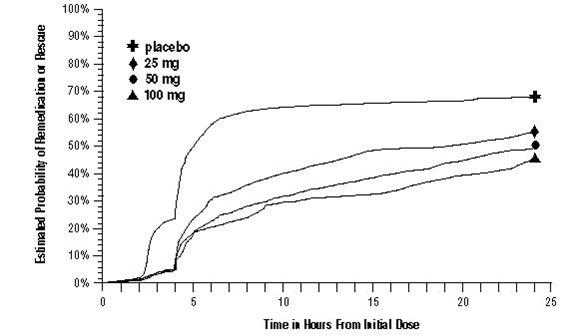
a Kaplan-Meier plot based on data obtained in the 3 clinical controlled trials providing evidence of efficacy with patients not using additional treatments censored to 24 hours. Plot also includes patients who had no response to the initial dose. No remedication was allowed within 2 hours postdose.
There is evidence that doses above 50 mg do not provide a greater effect than 50 mg. There was no evidence to suggest that treatment with sumatriptan tablets was associated with an increase in the severity of recurrent headaches. The efficacy of sumatriptan tablets was unaffected by presence of aura; duration of headache prior to treatment; gender, age, or weight of the subject; relationship to menses; or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants). There were insufficient data to assess the impact of race on efficacy.