Tacrolimus Prescribing Information
- Increased risk for developing serious infections and malignancies with tacrolimus or other immunosuppressants that may lead to hospitalization or death. (
5.1 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including tacrolimus, are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, examine patients for skin changes; exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) has been reported in immunosuppressed organ transplant recipients. The majority of PTLD events appear related to Epstein-Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children. Monitor EBV serology during treatment.
,5.2 Serious InfectionsPatients receiving immunosuppressants, including tacrolimus, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes. Serious viral infections reported include:
• Polyomavirus-associated nephropathy (PVAN), mostly due to BK virus infection
• JC virus-associated progressive multifocal leukoencephalopathy (PML)
• Cytomegalovirus infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor disease are at higher risk of developing CMV viremia and CMV disease.
Monitor for the development of infection and adjust the immunosuppressive regimen to balance the risk of rejection with the risk of infection [see Adverse Reactions ( 6.1,
6.2)].)
Warnings and Precautions (
Tacrolimus, like other calcineurin inhibitors, can cause acute or chronic nephrotoxicity. in transplant patients due to its vasoconstrictive effect on renal vasculature, toxic tubulopathy and tubular-interstitial effects. Nephrotoxicity was reported in clinical trials
Tacrolimus capsules are not recommended for use with sirolimus:
- The use of sirolimus with tacrolimus in studies of de novo liver transplant patients was associated with an excess mortality, graft loss, and hepatic artery thrombosis (HAT) and is not recommended.
- The use of sirolimus (2 mg per day) with tacrolimus in heart transplant patients in a U.S. trial was associated with increased risk of renal function impairment, wound healing complications, and insulin-dependent post-transplant diabetes mellitus, and is not recommended[see Clinical Studies ].
- The use of sirolimus with tacrolimus capsules may increase the risk of thrombotic microangiopathy[see Warnings and Precautions ].
Cases of thrombotic microangiopathy (TMA), including hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP), have been reported in patients treated with tacrolimus capsules. TMA may have a multifactorial etiology. Risk factors for TMA that can occur in transplant patients include, for example, severe infections, graft-versus-host disease (GVHD), Human Leukocyte Antigen (HLA) mismatch, the use of calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors. These risk factors may, either alone or combined, contribute to the risk of TMA.
In patients with signs and symptoms of TMA, consider tacrolimus as a risk factor. Concurrent use of tacrolimus and mTOR inhibitors may contribute to the risk of TMA.
Tacrolimus Capsules USP, 0.5 mg are white to off white powder filled in hard gelatin capsule of size ‘4’, dark yellow opaque cap imprinted with ‘0.5 MG’ and dark yellow opaque body imprinted with ‘RDY 525’using red ink.
Tacrolimus Capsules USP, 1 mg are white to off white powder filled in hard gelatin capsule of size ‘4’, white opaque cap imprinted with ‘1 MG’ and white opaque body imprinted with ‘RDY 526’using red ink.
Tacrolimus Capsules USP, 5 mg are white to off white powder filled in hard gelatin capsule of size ‘4’, dark grayish red opaque cap imprinted with ‘5 MG’ and dark grayish red opaque body imprinted with ‘RDY 527’ using white ink.
Tacrolimus capsules are contraindicated in patients with a hypersensitivity to tacrolimus. Tacrolimus injection is contraindicated in patients with a hypersensitivity to HCO-60 (polyoxyl 60 hydrogenated castor oil). Hypersensitivity symptoms reported include dyspnea, rash, pruritus, and acute respiratory distress syndrome
The following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling:
- Lymphoma and Other Malignancies [seeWarnings and Precautions(5.1)]
- Serious Infections [seeWarnings and Precautions]
- New Onset Diabetes After Transplant [seeWarnings and Precautions]
- Nephrotoxicity [seeWarnings and Precautions]
- Neurotoxicity [seeWarnings and Precautions]
- Hyperkalemia [seeWarnings and Precautions]
- Hypertension [seeWarnings and Precautions]
- Anaphylactic Reactions with Tacrolimus Injection [seeWarnings and Precautions]
- Myocardial Hypertrophy [seeWarnings and Precautions]
- Pure Red Cell Aplasia [seeWarnings and Precautions]
- Thrombotic Microangiopathy, Including Hemolytic Uremic Syndrome and Thrombotic Thrombocytopenic Purpura[see Warnings and Precautions ( 5.16)]
The most common adverse reactions (≥ 15%) were abnormal renal function, hypertension, diabetes mellitus, fever, CMV infection, tremor, hyperglycemia, leukopenia, infection, anemia, bronchitis, pericardial effusion, urinary tract infection, constipation, diarrhea, headache, abdominal pain, insomnia, paresthesia, peripheral edema, nausea, hyperkalemia, hypomagnesemia, and hyperlipemia. (6.1)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In addition, the clinical trials were not designed to establish comparative differences across study arms with regards to the adverse reactions discussed below.
The incidence of adverse reactions was determined in three randomized kidney transplant trials. One of the trials used azathioprine (AZA) and corticosteroids and two of the trials used mycophenolate mofetil (MMF) and corticosteroids concomitantly for maintenance immunosuppression.
Tacrolimus-based immunosuppression in conjunction with azathioprine and corticosteroids following kidney transplantation was assessed in a trial where 205 patients received tacrolimus-based immunosuppression and 207 patients received cyclosporine-based immunosuppression. The trial population had a mean age of 43 years (mean±SD was 43±13 years on tacrolimus and 44±12 years on cyclosporine arm), the distribution was 61% male, and the composition was White (58%), African-American (25%), Hispanic (12%), and Other (5%). The 12 month post-transplant information from this trial is presented below.
The most common adverse reactions (≥ 30%) observed in tacrolimus-treated kidney transplant patients are: infection, tremor, hypertension, abnormal renal function, constipation, diarrhea, headache, abdominal pain, insomnia, nausea, hypomagnesemia, urinary tract infection, hypophosphatemia, peripheral edema, asthenia, pain, hyperlipidemia, hyperkalemia, and anemia. Based on reported adverse reaction terms related to decreased renal function, nephrotoxicity was reported in approximately 52% of kidney transplantation patients.
Adverse reactions that occurred in ≥ 15% of kidney transplant patients treated with tacrolimus in conjunction with azathioprine are presented below:
Tacrolimus/AZA (N=205) | Cyclosporine/AZA (N=207) | |
Nervous System Tremor Headache Insomnia Paresthesia Dizziness | 54% 44% 32% 23% 19% | 34% 38% 30% 16% 16% |
Gastrointestinal | ||
| Diarrhea Nausea Constipation Vomiting Dyspepsia | 44% 38% 35% 29% 28% | 41% 36% 43% 23% 20% |
Cardiovascular | ||
| Hypertension Chest Pain | 50% 19% | 52% 13% |
Urogenital | ||
| Creatinine Increased Urinary Tract Infection | 45% 34% | 42% 35% |
Metabolic and Nutritional | ||
| Hypophosphatemia Hypomagnesemia Hyperlipemia Hyperkalemia Diabetes Mellitus Hypokalemia Hyperglycemia Edema | 49% 34% 31% 31% 24% 22% 22% 18% | 53% 17% 38% 32% 9% 25% 16% 19% |
Hemic and Lymphatic | ||
| Anemia Leukopenia | 30% 15% | 24% 17% |
Miscellaneous | ||
| Infection | 45% | 49% |
| Peripheral Edema Asthenia Abdominal Pain Pain Fever Back Pain | 36% 34% 33% 32% 29% 24% | 48% 30% 31% 30% 29% 20% |
Respiratory System | ||
| Dyspnea Cough Increased | 22% 18% | 18% 15% |
Musculoskeletal | ||
| Arthralgia | 25% | 24% |
Skin | ||
| Rash Pruritus | 17% 15% | 12% 7% |
Two trials were conducted for tacrolimus-based immunosuppression in conjunction with MMF and corticosteroids. In the non-US trial (Study 1), the incidence of adverse reactions was based on 1195 kidney transplant patients that received tacrolimus (Group C, n=403), or one of two cyclosporine (CsA) regimens (Group A, n=384 and Group B, n=408) in combination with MMF and corticosteroids; all patients, except those in one of the two cyclosporine groups, also received induction with daclizumab. The trial population had a mean age of 46 years (range 17 to 76); the distribution was 65% male, and the composition was 93% Caucasian. The 12 month post-transplant information from this trial is presented below.
Adverse reactions that occurred in ≥ 10% of kidney transplant patients treated with tacrolimus in conjunction with MMF in Study 1 [Note: This trial was conducted entirely outside of the United States. Such trials often report a lower incidence of adverse reactions in comparison to U.S. trials] are presented below:
Tacrolimus | Cyclosporine | Cyclosporine | |
(Group C) | (Group A) | (Group B) | |
(N=403) | (N=384) | (N=408) | |
| Diarrhea | 25% | 16% | 13% |
| Urinary Tract Infection | 24% | 28% | 24% |
| Anemia | 17% | 19% | 17% |
| Hypertension | 13% | 14% | 12% |
| Leukopenia | 13% | 10% | 10% |
| Edema Peripheral | 11% | 12% | 13% |
| Hyperlipidemia | 10% | 15% | 13% |
| Key: Group A = CsA/MMF/CS, B = CsA/MMF/CS/Daclizumab, C = Tac/MMF/CS/Daclizumab CsA = Cyclosporine, CS = Corticosteroids, Tac = Tacrolimus, MMF = mycophenolate mofetil | |||
In the U.S. trial (Study 2) with tacrolimus-based immunosuppression in conjunction with MMF and corticosteroids, 424 kidney transplant patients received tacrolimus (n=212) or cyclosporine (n=212) in combination with MMF 1 gram twice daily, basiliximab induction, and corticosteroids. The trial population had a mean age of 48 years (range 17 to 77); the distribution was 63% male, and the composition was White (74%), African-American (20%), Asian (3%), and Other (3%). The 12-month post-transplant information from this trial is presented below.
Adverse reactions that occurred in ≥15% of kidney transplant patients treated with tacrolimus in conjunction with MMF in Study 2 are presented below:
Tacrolimus/MMF | Cyclosporine/MMF | |
(N=212) | (N=212) | |
Gastrointestinal Disorders | ||
| Diarrhea | 44% | 26% |
| Nausea | 39% | 47% |
| Constipation | 36% | 41% |
| Vomiting | 26% | 25% |
| Dyspepsia | 18% | 15% |
Injury, Poisoning, and Procedural Complications | ||
| Post-Procedural Pain | 29% | 27% |
| Incision Site Complication | 28% | 23% |
| Graft Dysfunction | 24% | 18% |
Metabolism and Nutrition Disorders | ||
| Hypomagnesemia | 28% | 22% |
| Hypophosphatemia | 28% | 21% |
| Hyperkalemia | 26% | 19% |
| Hyperglycemia | 21% | 15% |
| Hyperlipidemia | 18% | 25% |
| Hypokalemia | 16% | 18% |
Nervous System Disorders | ||
| Tremor | 34% | 20% |
| Headache | 24% | 25% |
Blood and Lymphatic System Disorders | ||
| Anemia | 30% | 28% |
| Leukopenia | 16% | 12% |
Miscellaneous | ||
| Edema Peripheral | 35% | 46% |
| Hypertension | 32% | 35% |
| Insomnia | 30% | 21% |
| Urinary Tract Infection | 26% | 22% |
| Blood Creatinine Increased | 23% | 23% |
Less frequently observed adverse reactions in kidney transplantation patients are described under the subsection "Less Frequently Reported Adverse Reactions (> 3% and < 15%) in Liver, Kidney, and Heart Transplant Studies.”
There were two randomized comparative liver transplant trials. In the U.S. trial, 263 adult and pediatric patients received tacrolimus and steroids and 266 patients received cyclosporine-based immunosuppressive regimen (CsA/AZA). The trial population had a mean age of 44 years (range 0.4 to 70); the distribution was 52% male, and the composition was White (78%), African-American (5%), Asian (2%), Hispanic (13%), and Other (2%). In the European trial, 270 patients received tacrolimus and steroids and 275 patients received CsA/AZA. The trial population had a mean age of 46 years (range 15 to 68); the distribution was 59% male, and the composition was White (95.4%), Black (1%), Asian (2%), and Other (2%).
The proportion of patients reporting more than one adverse event was > 99% in both the tacrolimus group and the CsA/AZA group. Precautions must be taken when comparing the incidence of adverse reactions in the U.S. trial to that in the European trial. The 12 month post-transplant information from the U.S. trial and from the European trial is presented below. The two trials also included different patient populations and patients were treated with immunosuppressive regimens of differing intensities. Adverse reactions reported in ≥15% in tacrolimus patients (combined trial results) are presented below for the two controlled trials in liver transplantation.
The most common adverse reactions (≥ 38%) observed in tacrolimus-treated liver transplant patients are: tremor, headache, diarrhea, hypertension, nausea, abnormal renal function, abdominal pain, insomnia, paresthesia, anemia, pain, fever, asthenia, hyperkalemia, hypomagnesemia, and hyperglycemia. These all occur with oral and IV administration of tacrolimus and some may respond to a reduction in dosing (e.g., tremor, headache, paresthesia, hypertension). Diarrhea was sometimes associated with other gastrointestinal complaints such as nausea and vomiting. Based on reported adverse reaction terms related to decreased renal function, nephrotoxicity was reported in approximately 40% and 36% of liver transplantation patients receiving tacrolimus in the U.S. and European randomized trials.
U.S. TRIAL | EUROPEAN TRIAL | |||
T acrolimus )(N=250 | Cyclosporine/AZA (N=250) | Tacrolimus (N=264) | Cyclosporine/AZA (N=265) | |
Nervous System | ||||
| Headache | 64% | 60% | 37% | 26% |
| Insomnia | 64% | 68% | 32% | 23% |
| Tremor | 56% | 46% | 48% | 32% |
| Paresthesia | 40% | 30% | 17% | 17% |
Gastrointestinal | ||||
| Diarrhea | 72% | 47% | 37% | 27% |
| Nausea | 46% | 37% | 32% | 27% |
LFT Abnormal | 36% | 30% | 6% | 5% |
| Anorexia | 34% | 24% | 7% | 5% |
| Vomiting | 27% | 15% | 14% | 11% |
| Constipation | 24% | 27% | 23% | 21% |
Cardiovascular | ||||
| Hypertension | 47% | 56% | 38% | 43% |
Urogenital | ||||
| Kidney Function Abnormal | 40% | 27% | 36% | 23% |
| Creatinine Increased | 39% | 25% | 24% | 19% |
| BUN Increased | 30% | 22% | 12% | 9% |
| Oliguria | 18% | 15% | 19% | 12% |
| Urinary Tract Infection | 16% | 18% | 21% | 19% |
Metabolic and Nutritional | ||||
| Hypomagnesemia | 48% | 45% | 16% | 9% |
| Hyperglycemia | 47% | 38% | 33% | 22% |
| Hyperkalemia | 45% | 26% | 13% | 9% |
| Hypokalemia | 29% | 34% | 13% | 16% |
Hemic and Lymphatic | ||||
| Anemia | 47% | 38% | 5% | 1% |
| Leukocytosis | 32% | 26% | 8% | 8% |
| Thrombocytopenia | 24% | 20% | 14% | 19% |
Miscellaneous | ||||
| Pain | 63% | 57% | 24% | 22% |
| Abdominal Pain | 59% | 54% | 29% | 22% |
| Asthenia | 52% | 48% | 11% | 7% |
| Fever | 48% | 56% | 19% | 22% |
| Back Pain | 30% | 29% | 17% | 17% |
| Ascites | 27% | 22% | 7% | 8% |
| Peripheral Edema | 26% | 26% | 12% | 14% |
Respiratory System | ||||
| Pleural Effusion | 30% | 32% | 36% | 35% |
| Dyspnea | 29% | 23% | 5% | 4% |
| Atelectasis | 28% | 30% | 5% | 4% |
Skin and Appendages | ||||
| Pruritus | 36% | 20% | 15% | 7% |
| Rash | 24% | 19% | 10% | 4% |
Less frequently observed adverse reactions in liver transplantation patients are described under the subsection “Less Frequently Reported Adverse Reactions (> 3% and < 15%) in Liver, Kidney, and Heart Transplant Studies.”
The incidence of adverse reactions was determined based on two trials in primary orthotopic heart transplantation. In a trial conducted in Europe, 314 patients received a regimen of antibody induction, corticosteroids, and azathioprine (AZA) in combination with tacrolimus (n=157) or cyclosporine (n=157) for 18 months. The trial population had a mean age of 51 years (range 18 to 65); the distribution was 82% male, and the composition was White (96%), Black (3%), and Other (1%).
The most common adverse reactions (≥ 15%) observed in tacrolimus-treated heart transplant patients are: abnormal renal function, hypertension, diabetes mellitus, CMV infection, tremor, hyperglycemia, leukopenia, infection, anemia, bronchitis, pericardial effusion, urinary tract infection, and hyperlipemia. Based on reported adverse reaction terms related to decreased renal function, nephrotoxicity was reported in approximately 59% of heart transplantation patients in the European trial.
Adverse reactions in heart transplant patients in the European trial are presented below:
Tacrolimus/AZA (N=157) | Cyclosporine/AZA (N=157) | |
Cardiovascular System | ||
| Hypertension | 62% | 69% |
| Pericardial Effusion | 15% | 14% |
| Body as a Whole | ||
| CMV Infection | 32% | 30% |
| Infection | 24% | 21% |
Metabolic and Nutritional Disorders | ||
| Diabetes Mellitus | 26% | 16% |
| Hyperglycemia | 23% | 17% |
| Hyperlipemia | 18% | 27% |
Hemic and Lymphatic System | ||
| Anemia | 50% | 36% |
| Leukopenia | 48% | 39% |
| Urogenital System | ||
| Kidney Function Abnormal | 56% | 57% |
| Urinary Tract Infection | 16% | 12% |
Respiratory System | ||
| Bronchitis | 17% | 18% |
Nervous System | ||
| Tremor | 15% | 6% |
In the European trial, the cyclosporine trough concentrations were above the pre-defined target range (i.e., 100 to 200 ng/mL) at Day 122 and beyond in 32% to 68% of the patients in the cyclosporine treatment arm, whereas the tacrolimus trough concentrations were within the pre-defined target range (i.e., 5 to 15 ng/mL) in 74% to 86% of the patients in the tacrolimus treatment arm.
In a U.S. trial, the incidence of adverse reactions was based on 331 heart transplant patients that received corticosteroids and tacrolimus in combination with sirolimus (n=109), tacrolimus in combination with MMF (n=107) or cyclosporine modified in combination with MMF (n=115) for 1 year. The trial population had a mean age of 53 years (range 18 to 75); the distribution was 78% male, and the composition was White (83%), African-American (13%) and Other (4%).
Only selected targeted treatment-emergent adverse reactions were collected in the U.S. heart transplantation trial. Those reactions that were reported at a rate of 15% or greater in patients treated with tacrolimus and MMF include the following: any target adverse reactions (99%), hypertension (89%), hyperglycemia requiring antihyperglycemic therapy (70%), hypertriglyceridemia (65%), anemia (hemoglobin <10 g/dL) (65%), fasting blood glucose >140 mg/dL (on two separate occasions) (61%), hypercholesterolemia (57%), hyperlipidemia (34%), WBCs <3,000 cells/mcL (34%), serious bacterial infections (30%), magnesium <1.2 mEq/L (24%), platelet count <75,000 cells/mcL (19%), and other opportunistic infections (15%).
Other targeted treatment-emergent adverse reactions in tacrolimus-treated patients occurred at a rate of less than 15%, and include the following: Cushingoid features, impaired wound healing, hyperkalemia,
New Onset Diabetes After Transplant (NODAT) is defined as a composite of fasting plasma glucose ≥126 mg/dL, HbA1C≥ 6%, insulin use ≥ 30 days, or oral hypoglycemic use. In a trial in kidney transplant patients (Study 2), NODAT was observed in 75% in the tacrolimus-treated and 61% in the NEORAL-treated patients without pre-transplant history of diabetes mellitus (Table 10) [see
Parameter | Treatment Group | |
Tacrolimus/MMF (N = 212) | NEORAL/MMF (N = 212) | |
| NODAT | 112/150 (75%) | 93/152 (61%) |
| Fasting Plasma Glucose ≥ 126 mg/dL | 96/150 (64%) | 80/152 (53%) |
| HbA1C≥ 6% | 59/150 (39%) | 28/152 (18%) |
| Insulin Use ≥ 30 days | 9/150 (6%) | 4/152 (3%) |
| Oral Hypoglycemic Use | 15/150 (10%) | 5/152 (3%) |
In early trials of tacrolimus, Post-Transplant Diabetes Mellitus (PTDM) was evaluated with a more limited criterion of “use of insulin for 30 or more consecutive days with < 5 day gap” in patients without a prior history of insulin-dependent diabetes mellitus or non-insulin dependent diabetes mellitus. Data are presented in Tables 11 to 14. PTDM was reported in 20% of Tacrolimus/Azathioprine (AZA)-treated kidney transplant patients without pre-transplant history of diabetes mellitus in a Phase 3 trial (Table 11). The median time to onset of PTDM was 68 days. Insulin dependence was reversible in 15% of these PTDM patients at one year and in 50% at 2 years post-transplant. African-American and Hispanic kidney transplant patients were at an increased risk of development of PTDM (Table 12).
Status of PTDM * | Tacrolimus/AZA | CsA/AZA |
| Patients without pre-transplant history of diabetes mellitus | 151 | 151 |
| New onset PTDM*, 1stYear | 30/151 (20%) | 6/151 (4%) |
| Still insulin-dependent at one year in those without prior history of diabetes | 25/151 (17%) | 5/151 (3%) |
| New onset PTDM*post 1 year | 1 | 0 |
| Patients with PTDM*at 2 years | 16/151 (11%) | 5/151 (3%) |
* Use of insulin for 30 or more consecutive days, with < 5-day gap, without a prior history of insulin-dependent diabetes mellitus or non-insulin dependent diabetes mellitus.
Patient Race | Patients Who Developed PTDM* | |
Tacrolimus | Cyclosporine | |
| African-American | 15/41 (37%) | 3 (8%) |
| Hispanic | 5/17 (29%) | 1 (6%) |
| Caucasian | 10/82 (12%) | 1 (1%) |
| Other | 0/11 (0%) | 1 (10%) |
| Total | 30/151 (20%) | 6 (4%) |
* Use of insulin for 30 or more consecutive days, with < 5-day gap, without a prior history of insulin-dependent diabetes mellitus or non-insulin dependent diabetes mellitus.
Insulin-dependent PTDM was reported in 18% and 11% of tacrolimus-treated liver transplant patients and was reversible in 45% and 31% of these patients at 1 year post-transplant, in the U.S. and European randomized trials, respectively, (Table 13). Hyperglycemia was associated with the use of tacrolimus in 47% and 33% of liver transplant recipients in the U.S. and European randomized trials, respectively, and may require treatment
Status of PTDM * | U.S. Trial | European Trial | ||
Tacrolimus | Cyclosporine | Tacrolimus | Cyclosporine | |
| Patients at risk† | 239 | 236 | 239 | 249 |
| New Onset PTDM* | 42 (18%) | 30 (13%) | 26 (11%) | 12 (5%) |
| Patients still on insulin at 1 year | 23 (10%) | 19 (8%) | 18 (8%) | 6 (2%) |
* Use of insulin for 30 or more consecutive days, with < 5-day gap, without a prior history of insulin-dependent diabetes mellitus or non-insulin dependent diabetes mellitus.
†Patients without pre-transplant history of diabetes mellitus.
Insulin-dependent PTDM was reported in 13% and 22% of tacrolimus-treated heart transplant patients receiving mycophenolate mofetil (MMF) or azathioprine (AZA) and was reversible in 30% and 17% of these patients at one year post-transplant, in the U.S. and European randomized trials, respectively (Table 14). Hyperglycemia, defined as two fasting plasma glucose levels ≥126 mg/dL, was reported with the use of tacrolimus plus MMF or AZA in 32% and 35% of heart transplant recipients in the U.S. and European randomized trials, respectively, and may require treatment [see
Status of PTDM* | U.S. Trial | European Trial | ||
Tacrolimus/ MMF | Cyclosporine/MMF | Tacrolimus/ AZA | Cyclosporine/ AZA | |
| Patients at risk† | 75 | 83 | 132 | 138 |
| New Onset PTDM* | 10 (13%) | 6 (7%) | 29 (22%) | 5 (4%) |
| Patients still on insulin at 1 year‡ | 7 (9%) | 1 (1%) | 24 (18%) | 4 (3%) |
*Use of insulin for 30 or more consecutive days without a prior history of insulin-dependent diabetes mellitus or non-insulin dependent diabetes mellitus.
†Patients without pre-transplant history of diabetes mellitus.
‡7 to 12 months for the U.S. trial.
The following adverse reactions were reported in either liver, kidney, and/or heart transplant recipients who were treated with tacrolimus in clinical trials.
Nervous System
:Abnormal dreams, agitation, amnesia, anxiety, confusion, convulsion, crying, depression, elevated mood, emotional lability, encephalopathy, hemorrhagic stroke, hallucinations, hypertonia, incoordination, monoparesis, myoclonus, nerve compression, nervousness, neuralgia, neuropathy, paralysis flaccid, psychomotor skills impaired, psychosis, quadriparesis, somnolence, thinking abnormal, vertigo, writing impairedSpecial Senses: Abnormal vision, amblyopia, ear pain, otitis media, tinnitus
Gastrointestinal: Cholangitis, cholestatic jaundice, duodenitis, dysphagia, esophagitis, flatulence, gastritis, gastroesophagitis, gastrointestinal hemorrhage, GGT increase, GI disorder, GI perforation, hepatitis, hepatitis granulomatous, ileus, increased appetite, jaundice, liver damage, esophagitis ulcerative, oral moniliasis, pancreatic pseudocyst, stomatitis
Cardiovascular: Abnormal ECG, angina pectoris, arrhythmia, atrial fibrillation, atrial flutter, bradycardia, cardiac fibrillation, cardiopulmonary failure, congestive heart failure, deep thrombophlebitis, echocardiogram abnormal, electrocardiogram QRS complex abnormal, electrocardiogram ST segment abnormal, heart failure, heart rate decreased, hemorrhage, hypotension, phlebitis, postural hypotension, syncope, tachycardia, thrombosis, vasodilatation
Urogenital: Acute kidney failure, albuminuria, BK nephropathy, bladder spasm, cystitis, dysuria, hematuria, hydronephrosis, kidney failure, kidney tubular necrosis, nocturia, pyuria, toxic nephropathy, urge incontinence, urinary frequency, urinary incontinence, urinary retention, vaginitis
Metabolic/Nutritional: Acidosis, alkaline phosphatase increased, alkalosis, ALT (SGPT) increased, AST (SGOT) increased, bicarbonate decreased, bilirubinemia, dehydration, GGT increased, gout, healing abnormal, hypercalcemia, hypercholesterolemia, hyperphosphatemia, hyperuricemia, hypervolemia, hypocalcemia, hypoglycemia, hyponatremia, hypoproteinemia, lactic dehydrogenase increased, weight gain
Endocrine: Cushing’s syndrome
Hemic/Lymphatic: Coagulation disorder, ecchymosis, hematocrit increased, hypochromic anemia, leukocytosis, polycythemia, prothrombin decreased, serum iron decreased
Miscellaneous: Abdomen enlarged, abscess, accidental injury, allergic reaction, cellulitis, chills, fall, flu syndrome, generalized edema, hernia, mobility decreased, peritonitis, photosensitivity reaction, sepsis, temperature intolerance, ulcer
Musculoskeletal: Arthralgia, cramps, generalized spasm, leg cramps, myalgia, myasthenia, osteoporosis
Respiratory: Asthma, emphysema, hiccups, lung function decreased, pharyngitis, pneumonia, pneumothorax, pulmonary edema, rhinitis, sinusitis, voice alteration
Skin: Acne, alopecia, exfoliative dermatitis, fungal dermatitis, herpes simplex, herpes zoster, hirsutism, neoplasm skin benign, skin discoloration, skin ulcer, sweating
Additional pediatric use information is approved for Astellas Pharma US, Inc.’s Prograf (tacrolimus) products. However, due to Astellas Pharma US, Inc.’s marketing exclusivity rights, this drug product is not labeled with that information
The following adverse reactions have been reported from worldwide marketing experience with tacrolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of the reporting, or (3) strength of causal connection to the drug.
Other reactions include:
Cardiovascular: Atrial fibrillation, atrial flutter, cardiac arrhythmia, cardiac arrest, electrocardiogram T wave abnormal, flushing, myocardial infarction, myocardial ischemia, pericardial effusion, QT prolongation,
Torsades de pointes, venous thrombosis deep limb, ventricular extrasystoles, ventricular fibrillation, myocardial hypertrophyGastrointestinal: Bile duct stenosis, colitis, enterocolitis, gastroenteritis, gastrooesophageal reflux disease, hepatic cytolysis, hepatic necrosis, hepatotoxicity, impaired gastric emptying, liver fatty, mouth ulceration, pancreatitis hemorrhagic, pancreatitis necrotizing, stomach ulcer, veno-occlusive liver disease
Hemic/Lymphatic: Agranulocytosis, disseminated intravascular coagulation, hemolytic anemia, neutropenia, febrile neutropenia, pancytopenia, thrombocytopenic purpura, thrombotic thrombocytopenic purpura, pure red cell aplasia thrombotic microangiopathy
Infections: Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal; -polyoma virus-associated nephropathy (PVAN) including graft loss
Metabolic/Nutritional: Glycosuria, increased amylase including pancreatitis, weight decreased
Miscellaneous: Feeling hot and cold, feeling jittery, hot flushes, multi-organ failure, primary graft dysfunction
Musculoskeletal and Connective Tissue Disorders: Pain in extremity including Calcineurin-Inhibitor Induced Pain Syndrome (CIPS)
Nervous System: Carpal tunnel syndrome, cerebral infarction, hemiparesis, leukoencephalopathy, mental disorder, mutism, posterior reversible encephalopathy syndrome (PRES), progressive multifocal leukoencephalopathy (PML), quadriplegia, speech disorder, syncope
Respiratory: Acute respiratory distress syndrome, interstitial lung disease, lung infiltration, respiratory distress, respiratory failure
Skin: Stevens-Johnson syndrome, toxic epidermal necrolysis
Special Senses: Blindness, optic neuropathy, blindness cortical, hearing loss including deafness, photophobia
Urogenital: Acute renal failure, cystitis hemorrhagic, hemolytic-uremic syndrome
The following serious and otherwise important adverse drug reactions are discussed in greater detail in other sections of labeling:
- Lymphoma and Other Malignancies [see Warnings and Precautions]
5.1 Lymphoma and Other MalignanciesPatients receiving immunosuppressants, including tacrolimus, are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, examine patients for skin changes; exposure to sunlight and UV light should be limited by wearing protective clothing and using a broad-spectrum sunscreen with a high protection factor.
Post-transplant lymphoproliferative disorder (PTLD) has been reported in immunosuppressed organ transplant recipients. The majority of PTLD events appear related to Epstein-Barr Virus (EBV) infection. The risk of PTLD appears greatest in those individuals who are EBV seronegative, a population which includes many young children. Monitor EBV serology during treatment.
- Serious Infections [see Warnings and Precautions(
5.2 Serious InfectionsPatients receiving immunosuppressants, including tacrolimus, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. These infections may lead to serious, including fatal, outcomes. Serious viral infections reported include:
• Polyomavirus-associated nephropathy (PVAN), mostly due to BK virus infection
• JC virus-associated progressive multifocal leukoencephalopathy (PML)
• Cytomegalovirus infections: CMV seronegative transplant patients who receive an organ from a CMV seropositive donor disease are at higher risk of developing CMV viremia and CMV disease.
Monitor for the development of infection and adjust the immunosuppressive regimen to balance the risk of rejection with the risk of infection [see Adverse Reactions ].
Tacrolimus may cause a spectrum of neurotoxicities. The most severe neurotoxicities include posterior reversible encephalopathy syndrome (PRES), delirium, seizure and coma; others include tremors, paresthesias, headache, mental status changes, and changes in motor and sensory functions
Hyperkalemia has been reported with tacrolimus use. Serum potassium levels should be monitored. Careful consideration should be given prior to use of other agents also associated with hyperkalemia (e.g., potassium-sparing diuretics, ACE inhibitors, angiotensin receptor blockers) during tacrolimus therapy
Hypertension is a common adverse effect of tacrolimus therapy and may require antihypertensive therapy
Anaphylactic reactions have occurred with injectables containing castor oil derivatives, including tacrolimus, in a small percentage of patients (0.6%). The exact cause of these reactions is not known. Tacrolimus injection should be reserved for patients who are unable to take tacrolimus orally. Monitor patients for anaphylaxis when using the intravenous route of administration [see
Myocardial hypertrophy has been reported in infants, children, and adults, particularly those with high tacrolimus trough concentrations, and is generally manifested by echocardiographically demonstrated concentric increases in left ventricular posterior wall and interventricular septum thickness. This condition appears reversible in most cases following dose reduction or discontinuance of therapy. In patients who develop renal failure or clinical manifestations of ventricular dysfunction while receiving tacrolimus therapy, echocardiographic evaluation should be considered. If myocardial hypertrophy is diagnosed, dosage reduction or discontinuation of tacrolimus should be considered
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with tacrolimus. A mechanism for tacrolimus-induced PRCA has not been elucidated. All patients reported risk factors for PRCA such as parvovirus B19 infection, underlying disease, or concomitant medications associated with PRCA. If PRCA is diagnosed, discontinuation of tacrolimus should be considered
Cases of thrombotic microangiopathy (TMA), including hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP), have been reported in patients treated with tacrolimus capsules. TMA may have a multifactorial etiology. Risk factors for TMA that can occur in transplant patients include, for example, severe infections, graft-versus-host disease (GVHD), Human Leukocyte Antigen (HLA) mismatch, the use of calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors. These risk factors may, either alone or combined, contribute to the risk of TMA.
In patients with signs and symptoms of TMA, consider tacrolimus as a risk factor. Concurrent use of tacrolimus and mTOR inhibitors may contribute to the risk of TMA.
Cases of thrombotic microangiopathy (TMA), including hemolytic uremic syndrome (HUS) and thrombotic thrombocytopenic purpura (TTP), have been reported in patients treated with tacrolimus capsules. TMA may have a multifactorial etiology. Risk factors for TMA that can occur in transplant patients include, for example, severe infections, graft-versus-host disease (GVHD), Human Leukocyte Antigen (HLA) mismatch, the use of calcineurin inhibitors and mammalian target of rapamycin (mTOR) inhibitors. These risk factors may, either alone or combined, contribute to the risk of TMA.
In patients with signs and symptoms of TMA, consider tacrolimus as a risk factor. Concurrent use of tacrolimus and mTOR inhibitors may contribute to the risk of TMA.
Tacrolimus USP, previously known as FK506, is the active ingredient in tacrolimus capsules USP. Tacrolimus USP is a calcineurin-inhibitor immunosuppressant produced by
The chemical structure of tacrolimus is:
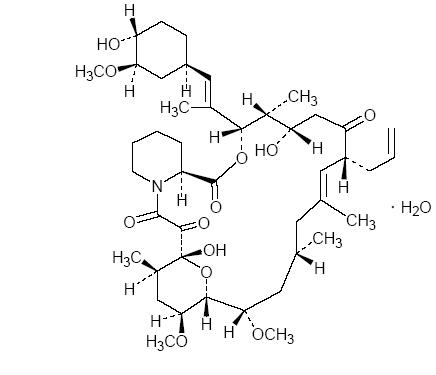
Tacrolimus USP has an molecular formula of C44H69NO12•H2O and a formula weight of 822.03. Tacrolimus USP appears as white to off white granular powder. It is practically insoluble in water, freely soluble in methanol, ethanol, acetone, ehyl acetate, chloroform.
Tacrolimus USP is available for oral administration as capsules containing the equivalent of 0.5 mg, 1 mg or 5 mg of anhydrous tacrolimus, USP. Inactive ingredients include croscarmellose sodium, lactose monohydrate and magnesium stearate. The 0.5 mg capsule shell contains gelatin, iron oxide red, iron oxide yellow and titanium dioxide, the 1 mg capsule shell contains gelatin and titanium dioxide and the 5 mg capsule shell contains gelatin, iron oxide red, iron oxide black, and titanium dioxide.
Tacrolimus Capsules meets USP Organic Impurities Test