Tacrolimus
Tacrolimus Prescribing Information
Tacrolimus Ointment, both 0.03% and 0.1% for adults, and only 0.03% for children aged 2 to 15 years, is indicated as
| WARNING |
|---|
Long-term Safety of Topical Calcineurin Inhibitors Has Not Been Established Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including Tacrolimus Ointment. Therefore:
|
Only the lower concentration, 0.03%, of Tacrolimus Ointment is recommended for use as a
The long-term safety and effects of Tacrolimus Ointment on the developing immune system are unknown (see boxed WARNING, WARNINGSand INDICATIONS and USAGE).
Four studies were conducted involving a total of about 4,400 patients 2-15 years of age: one 12-week randomized vehicle-controlled study and three open-label safety studies of one to three years duration. About 2,500 of these patients were 2 to 6 years of age.
The most common adverse events from these studies associated with Tacrolimus Ointment application in pediatric patients were skin burning and pruritus (see ADVERSE REACTIONS). In addition to skin burning and pruritus, the less common events (<5%) of varicella zoster (mostly chicken pox), and vesiculobullous rash were more frequent in patients treated with Tacrolimus Ointment 0.03% compared to vehicle. In the open-label safety studies, the incidence of adverse events, including infections, did not increase with increased duration of study drug exposure or amount of ointment used. In about 4,400 pediatric patients treated with Tacrolimus Ointment, 24 (0.5%) were reported with eczema herpeticum. Since the safety and efficacy of Tacrolimus Ointment have not been established in pediatric patients below 2 years of age, its use in this age group is not recommended.
In an open-label study, immune response to a 23-valent pneumococcal polysaccharide vaccine was assessed in 23 children 2 to 12 years old with moderate to severe atopic dermatitis treated with Tacrolimus Ointment 0.03%. Protective antibody titers developed in all patients. Similarly, in a seven-month, double-blind trial, the vaccination response to meningococcal serogroup C was equivalent in children 2 to 11 years old with moderate to severe atopic dermatitis treated with Tacrolimus Ointment 0.03% (n=121), a hydrocortisone ointment regimen (n=111), or normal children (n=44).
- Apply a thin layer of Tacrolimus Ointment to the affected skin twice daily. The minimum amount should be rubbed in gently and completely to control signs and symptoms of atopic dermatitis. Stop using when signs and symptoms of atopic dermatitis resolve.
- If signs and symptoms (e.g. itch, rash, and redness) do not improve within 6 weeks, patients should be re-examined by their healthcare provider to confirm the diagnosis of atopic dermatitis.
- Continuous long-term use of topical calcineurin inhibitors, including Tacrolimus Ointment should be avoided, and application should be limited to areas of involvement with atopic dermatitis.
The safety of Tacrolimus Ointment under occlusion, which may promote systemic exposure, has not been evaluated. Tacrolimus Ointment should not be used with occlusive dressings.
Tacrolimus Ointment is contraindicated in patients with a history of hypersensitivity to tacrolimus or any other component of the ointment.
No phototoxicity and no photoallergenicity were detected in clinical studies with 12 and 216 normal volunteers, respectively. One out of 198 normal volunteers showed evidence of sensitization in a contact sensitization study.
In three 12 week randomized vehicle-controlled studies and four safety studies, 655 and 9,163 patients respectively, were treated with Tacrolimus Ointment. The duration of follow-up for adult and pediatric patients in the safety studies is tabulated below.
| Time on Study | Adult | Pediatrics | Total |
|---|---|---|---|
| < 1 year | 4682 | 4481 | 9163 |
| ≥ 1 year | 1185 | 1349 | 2534 |
| ≥ 2 years | 200 | 275 | 475 |
| ≥ 3 years | 118 | 182 | 300 |
The following table depicts the adjusted incidence of adverse events pooled across the 3 identically designed 12-week controlled studies for patients in vehicle, Tacrolimus Ointment 0.03%, and Tacrolimus Ointment 0.1% treatment groups. The table also depicts the unadjusted incidence of adverse events in four safety studies, regardless of relationship to study drug.
| 12-Week, Randomized, Double-Blind, Phase 3 Studies 12-Week Adjusted Incidence Rate (%) | Open-Label Studies (up to 3 years) 0.1% and 0.03% Tacrolimus Ointment Incidence Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Adult | Pediatric | Adult | Pediatric | Total | ||||
| Vehicle (n=212) % | 0.03% Tacrolimus Ointment (n=210) % | 0.1% Tacrolimus Ointment (n=209) % | Vehicle (n=116) % | 0.03% Tacrolimus Ointment (n=118) % | (n=4682) % | (n=4481) % | (n=9163) % | |
| Skin BurningMay be reasonably associated with the use of this drug product | 26 | 46 | 58 | 29 | 43 | 28 | 20 | 24 |
| Pruritus | 37 | 46 | 46 | 27 | 41 | 25 | 19 | 22 |
| Flu-like symptoms | 19 | 23 | 31 | 25 | 28 | 22 | 34 | 28 |
| Allergic Reaction | 8 | 12 | 6 | 8 | 4 | 9 | 13 | 11 |
| Skin Erythema | 20 | 25 | 28 | 13 | 12 | 12 | 7 | 9 |
| Headache | 11 | 20 | 19 | 8 | 5 | 13 | 9 | 11 |
| Skin Infection | 11 | 12 | 5 | 14 | 10 | 9 | 16 | 12 |
| Fever | 4 | 4 | 1 | 13 | 21 | 2 | 14 | 8 |
| Infection | 1 | 1 | 2 | 9 | 7 | 6 | 10 | 8 |
| Cough Increased | 2 | 1 | 1 | 14 | 18 | 3 | 10 | 6 |
| Asthma | 4 | 6 | 4 | 6 | 6 | 4 | 13 | 8 |
| Herpes Simplex | 4 | 4 | 4 | 2 | 0 | 4 | 3 | 3 |
| Eczema Herpeticum | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 |
| Pharyngitis | 3 | 3 | 4 | 11 | 6 | 4 | 12 | 8 |
| Accidental Injury | 4 | 3 | 6 | 3 | 6 | 6 | 8 | 7 |
| Pustular Rash | 2 | 3 | 4 | 3 | 2 | 2 | 7 | 5 |
| Folliculitis | 1 | 6 | 4 | 0 | 2 | 4 | 2 | 3 |
| Rhinitis | 4 | 3 | 2 | 2 | 6 | 2 | 4 | 3 |
| Otitis Media | 4 | 0 | 1 | 6 | 12 | 2 | 11 | 6 |
| Sinusitis | 1 | 4 | 2 | 8 | 3 | 6 | 7 | 6 |
| Diarrhea | 3 | 3 | 4 | 2 | 5 | 2 | 4 | 3 |
| Urticaria | 3 | 3 | 6 | 1 | 1 | 3 | 4 | 4 |
| Lack of Drug Effect | 1 | 1 | 0 | 1 | 1 | 6 | 6 | 6 |
| Bronchitis | 0 | 2 | 2 | 3 | 3 | 4 | 4 | 4 |
| Vomiting | 0 | 1 | 1 | 7 | 6 | 1 | 4 | 3 |
| Maculopapular Rash | 2 | 2 | 2 | 3 | 0 | 2 | 1 | 1 |
| Rash | 1 | 5 | 2 | 4 | 2 | 2 | 3 | 3 |
| Abdominal Pain | 3 | 1 | 1 | 2 | 3 | 1 | 3 | 2 |
| Fungal Dermatitis | 0 | 2 | 1 | 3 | 0 | 2 | 4 | 3 |
| Gastroenteritis | 1 | 2 | 2 | 3 | 0 | 2 | 4 | 3 |
| Alcohol Intolerance | 0 | 3 | 7 | 0 | 0 | 4 | 0 | 2 |
| Acne | 2 | 4 | 7 | 1 | 0 | 3 | 2 | 3 |
| Sunburn | 1 | 2 | 1 | 0 | 0 | 2 | 1 | 1 |
| Skin Disorder | 2 | 2 | 1 | 1 | 4 | 2 | 2 | 2 |
| Conjunctivitis | 0 | 2 | 2 | 2 | 1 | 3 | 3 | 3 |
| Pain | 1 | 2 | 1 | 0 | 1 | 2 | 1 | 2 |
| Vesiculobullous Rash | 3 | 3 | 2 | 0 | 4 | 2 | 1 | 1 |
| Lymphadenopathy | 2 | 2 | 1 | 0 | 3 | 1 | 2 | 1 |
| Nausea | 4 | 3 | 2 | 0 | 1 | 2 | 1 | 2 |
| Skin Tingling | 2 | 3 | 8 | 1 | 2 | 2 | 1 | 1 |
| Face Edema | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 |
| Dyspepsia | 1 | 1 | 4 | 0 | 0 | 2 | 2 | 2 |
| Dry Skin | 7 | 3 | 3 | 0 | 1 | 1 | 1 | 1 |
| Hyperesthesia | 1 | 3 | 7 | 0 | 0 | 2 | 0 | 1 |
| Skin Neoplasm BenignGenerally "warts". | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 2 |
| Back Pain | 0 | 2 | 2 | 1 | 1 | 3 | 0 | 2 |
| Peripheral Edema | 2 | 4 | 3 | 0 | 0 | 2 | 0 | 1 |
| Varicella Zoster/Herpes Zoster All the herpes zoster cases in the pediatric 12-week study and the majority of cases in the open-label pediatric studies were reported as chicken pox. | 0 | 1 | 0 | 0 | 5 | 1 | 2 | 2 |
| Contact Dermatitis | 1 | 3 | 3 | 3 | 4 | 2 | 2 | 2 |
| Asthenia | 1 | 2 | 3 | 0 | 0 | 1 | 0 | 1 |
| Pneumonia | 0 | 1 | 1 | 2 | 0 | 1 | 3 | 2 |
| Eczema | 2 | 2 | 2 | 0 | 0 | 1 | 0 | 1 |
| Insomnia | 3 | 4 | 3 | 1 | 1 | 2 | 0 | 1 |
| Exfoliative Dermatitis | 3 | 3 | 1 | 0 | 0 | 0 | 1 | 0 |
| Dysmenorrhea | 2 | 4 | 4 | 0 | 0 | 2 | 1 | 1 |
| Periodontal Abscess | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| Myalgia | 0 | 3 | 2 | 0 | 0 | 2 | 1 | 1 |
| Cyst | 0 | 1 | 3 | 0 | 0 | 1 | 0 | 1 |
| Cellulitis | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Exacerbation of Untreated Area | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| Procedural Complication | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| Hypertension | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 1 |
| Tooth Disorder | 0 | 1 | 1 | 1 | 0 | 2 | 1 | 1 |
| Arthralgia | 1 | 1 | 3 | 2 | 0 | 2 | 1 | 2 |
| Depression | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 1 |
| Paresthesia | 1 | 3 | 3 | 0 | 0 | 2 | 1 | 2 |
| Alopecia | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Urinary Tract Infection | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 2 |
| Ear Pain | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
Other adverse events which occurred at an incidence between 0.2% and less than 1% in clinical studies in the above table include: abnormal vision, abscess, anaphylactoid reaction, anemia, anorexia, anxiety, arthritis, arthrosis, bilirubinemia, blepharitis, bone disorder, breast neoplasm benign, bursitis, cataract NOS, chest pain, chills, colitis, conjunctival edema, constipation, cramps, cutaneous moniliasis, cystitis, dehydration, dizziness, dry eyes, dry mouth/nose, dyspnea, ear disorder, ecchymosis, edema, epistaxis, eye pain, furunculosis, gastritis, gastrointestinal disorder, hernia, hypercholesterolemia, hypertonia, hypothyroidism, joint disorder, laryngitis, leukoderma, lung disorder, malaise, migraine, moniliasis, mouth ulceration, nail disorder, neck pain, neoplasm benign, oral moniliasis, otitis externa, photosensitivity reaction, rectal disorder, seborrhea, skin carcinoma, skin discoloration, skin hypertrophy, skin ulcer, stomatitis, tendon disorder, thinking abnormal, tooth caries, sweating, syncope, tachycardia, taste perversion, unintended pregnancy, vaginal moniliasis, vaginitis, valvular heart disease, vasodilatation, and vertigo.
Formal topical drug interaction studies with Tacrolimus Ointment have not been conducted. Based on its extent of absorption, interactions of Tacrolimus Ointment with systemically administered drugs are unlikely to occur but cannot be ruled out (see
The mechanism of action of tacrolimus in atopic dermatitis is not known. While the following have been observed, the clinical significance of these observations in atopic dermatitis is not known. It has been demonstrated that tacrolimus inhibits T-lymphocyte activation by first binding to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is then formed and the phosphatase activity of calcineurin is inhibited. This effect has been shown to prevent the dephosphorylation and translocation of nuclear factor of activated T-cells (NF-AT), a nuclear component thought to initiate gene transcription for the formation of lymphokines (such as interleukin-2, gamma interferon). Tacrolimus also inhibits the transcription for genes which encode IL-3, IL-4, IL-5, GM-CSF, and TNF-α, all of which are involved in the early stages of T-cell activation. Additionally, tacrolimus has been shown to inhibit the release of pre-formed mediators from skin mast cells and basophils, and to down regulate the expression of FcɛRI on Langerhans cells.
The pooled results from three pharmacokinetic studies in 88 adult atopic dermatitis patients indicate that tacrolimus is minimally absorbed after the topical application of Tacrolimus Ointment. Peak tacrolimus blood concentrations ranged from undetectable to 20 ng/mL after single or multiple doses of 0.03% and 0.1% Tacrolimus Ointment, with 85% (75/88) of the patients having peak blood concentrations less than 2 ng/mL. In general as treatment continued, systemic exposure declined as the skin returned to normal. In clinical studies with periodic blood sampling, a similar distribution of tacrolimus blood levels was also observed in adult patients, with 90% (1253/1391) of patients having a blood concentration less than 2 ng/mL.
The absolute bioavailability of tacrolimus from Tacrolimus Ointment in atopic dermatitis patients is approximately 0.5%. In adults with an average of 53% BSA treated, exposure (AUC) of tacrolimus from Tacrolimus Ointment is approximately 30-fold less than that seen with oral immunosuppressive doses in kidney and liver transplant patients.
Mean peak tacrolimus blood concentrations following oral administration (0.3 mg/kg/day) in adult kidney transplant (n=26) and liver transplant (n=17) patients are 24.2±15.8 ng/mL and 68.5±30.0 ng/mL, respectively. The lowest tacrolimus blood level at which systemic effects (e.g., immunosuppression) can be observed is not known.
Systemic levels of tacrolimus have also been measured in pediatric patients (see
The plasma protein binding of tacrolimus is approximately 99% and is independent of concentration over a range of 5-50 ng/mL. Tacrolimus is bound mainly to albumin and alpha-1-acid glycoprotein, and has a high level of association with erythrocytes. The distribution of tacrolimus between whole blood and plasma depends on several factors, such as hematocrit, temperature at the time of plasma separation, drug concentration, and plasma protein concentration. In a US study, the ratio of whole blood concentration to plasma concentration averaged 35 (range 12 to 67).
There was no evidence based on blood concentrations that tacrolimus accumulates systemically upon intermittent topical application for periods of up to 1 year. As with other topical calcineurin inhibitors, it is not known whether tacrolimus is distributed into the lymphatic system.
Tacrolimus is extensively metabolized by the mixed-function oxidase system, primarily the cytochrome P-450 system (CYP3A). A metabolic pathway leading to the formation of 8 possible metabolites has been proposed. Demethylation and hydroxylation were identified as the primary mechanisms of biotransformation in vitro. The major metabolite identified in incubations with human liver microsomes is 13-demethyl tacrolimus. In in vitro studies, a 31-demethyl metabolite has been reported to have the same activity as tacrolimus.
The mean clearance following IV administration of tacrolimus is 0.040, 0.083 and 0.053 L/hr/kg in healthy volunteers, adult kidney transplant patients and adult liver transplant patients, respectively. In man, less than 1% of the dose administered is excreted unchanged in urine.
In a mass balance study of IV administered radiolabeled tacrolimus to 6 healthy volunteers, the mean recovery of radiolabel was 77.8 ± 12.7%. Fecal elimination accounted for 92.4 ± 1.0% and the elimination half-life based on radioactivity was 48.1 ± 15.9 hours whereas it was 43.5 ± 11.6 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.029 ± 0.015 L/hr/kg and clearance of tacrolimus was 0.029 ± 0.009 L/hr/kg.
When administered PO, the mean recovery of the radiolabel was 94.9 ± 30.7%. Fecal elimination accounted for 92.6 ± 30.7%, urinary elimination accounted for 2.3 ± 1.1% and the elimination half-life based on radioactivity was 31.9 ± 10.5 hours whereas it was 48.4 ± 12.3 hours based on tacrolimus concentrations. The mean clearance of radiolabel was 0.226 ± 0.116 L/hr/kg and clearance of tacrolimus 0.172 ± 0.088 L/hr/kg.
In a pharmacokinetic study of 14 pediatric atopic dermatitis patients, between the ages of 2-5 years, peak blood concentrations of tacrolimus ranged from undetectable to 14.8 ng/mL after single or multiple doses of 0.03% Tacrolimus Ointment, with 86% (12/14) of patients having peak blood concentrations below 2 ng/mL throughout the study.
The highest peak concentration was observed in one patient with 82% BSA involvement on day 1 following application of 0.03% Tacrolimus Ointment. The peak concentrations for this subject were 14.8 ng/mL on day 1 and 4.1 ng/mL on day 14. Mean peak tacrolimus blood concentrations following oral administration in pediatric liver transplant patients (n = 9) were 48.4 ± 27.9 ng/mL.
In a similar pharmacokinetic study with 61 enrolled pediatric patients (ages 6-12 years) with atopic dermatitis, peak tacrolimus blood concentrations ranged from undetectable to 5.3 ng/mL after single or multiple doses of 0.1% Tacrolimus Ointment, with 91% (52/57) of evaluable patients having peak blood concentrations below 2 ng/mL throughout the study period. When detected, systemic exposure generally declined as treatment continued.
In clinical studies with periodic blood sampling, a similar distribution of tacrolimus blood levels was also observed, with 98% (509/522) of pediatric patients having a blood concentration below 2 ng/mL.
The effect of renal insufficiency on the pharmacokinetics of topically administered tacrolimus has not been evaluated. The mean clearance of IV administered tacrolimus in patients with renal dysfunction was similar to that of normal volunteers. On the basis of this information dose-adjustment is not expected to be needed.
The effect of hepatic insufficiency on the pharmacokinetics of topically administered tacrolimus has not been evaluated but dose-adjustment is not expected to be needed.
Tacrolimus Ointment contains tacrolimus, a macrolide immunosuppressant produced by
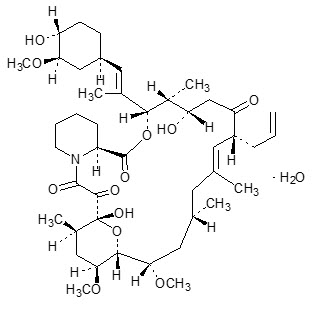
Tacrolimus has an empirical formula of C44H69NO12∙H2O and a formula weight of 822.03. Each gram of Tacrolimus Ointment contains (w/w) either 0.03% or 0.1% of tacrolimus in a base of mineral oil with all-