Tarpeyo
(Budesonide)Tarpeyo Prescribing Information
TARPEYO is indicated to reduce the loss of kidney function in adults with primary immunoglobulin A nephropathy (IgAN) who are at risk for disease progression.
The recommended duration of therapy is 9 months, with a dosage of 16 mg administered orally once daily
TARPEYO was shown to reduce the loss of kidney function in adults with primary IgAN at risk of disease progression in the NefIgArd trial. While the effect on kidney function that was seen during the 9-month treatment period persisted following completion of treatment, TARPEYO did not change the long-term rate of decline in kidney function.
The effect of TARPEYO on proteinuria and kidney function (estimated glomerular filtration rate, eGFR) was assessed in a randomized, double-blind, phase 3, 2-part, multicenter study (NefIgArd, NCT: 03643965) in adults with biopsy-proven IgAN, eGFR ≥35 mL/min/1.73 m2, and proteinuria (defined as either ≥1 g/day or urine protein to creatinine ratio (UPCR) ≥0.8 g/g) who were on a stable dose of maximally-tolerated RAS inhibitor therapy. Patients with other glomerulopathies, nephrotic syndrome, or those who had been treated with systemic immunosuppressive medications were excluded. Patients were randomized 1:1 to either TARPEYO 16 mg once daily or placebo and treated for nine months followed by a 2-week taper of either TARPEYO 8 mg once daily or placebo. Patients were then followed off-treatment for 15 months. The primary endpoint for Part A of the study (interim analysis) was the ratio of UPCR (based on 24-hour urine collections) at 9 months compared to baseline based on the first 199 randomized patients who completed the Month 9 visit. The primary endpoint for Part B of the study (final analysis) was a time-weighted average of the log ratio of eGFR at each time point over 2 years relative to baseline.
Of the 364 randomized patients evaluated for efficacy, 66% were male, 76% were Caucasian, 23% were Asian, and 20% were from North America. The median age was 43 years (range 20 to 73 years). At baseline, the mean eGFR was approximately 58 mL/min/1.73 m2, with 60% of patients having an eGFR <60 mL/min/1.73 m2. The mean baseline UPCR was 1.5 g/g and 21% of patients had proteinuria >3.5 g/24 hours. Approximately 70% of patients had a history of hypertension and 7% had a history of type 2 diabetes mellitus. At baseline, 98% were treated with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and <1% of patients were on a sodium-glucose cotransporter 2 (SGLT2) inhibitor. At study entry, the median systolic/diastolic blood pressure was 125/79 mmHg.
The trial met the prespecified Part A primary endpoint based on an interim analysis of 199 randomized patients who had completed the Month 9 visit. The interim analysis showed a 31% reduction in UPCR in patients treated with TARPEYO 16 mg once daily compared to placebo (95% CI: 16% to 42% reduction; p=0.0001). In the final analysis of 364 patients, the percentage change in UPCR observed at 9 months was consistent with the results in the subset of 199 patients included in the interim analysis. The final analysis of the percentage change in UPCR during the treatment and follow-up phase is shown in Figure 1.
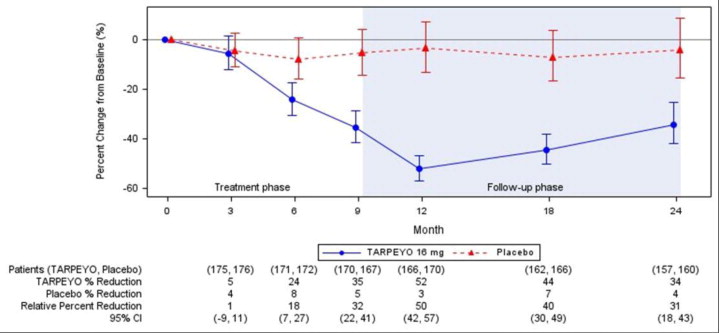
Estimated mean percentage change from baseline in UPCR with 95% confidence intervals estimated from a mixed model repeated measures analysis of log-transformed post-baseline to baseline ratios at 3, 6, 9, 12, 18, and 24 months. Analysis included all UPCR data regardless of use of prohibited medication at any point during the study.
Values reported under the figure are converted to percent reduction from baseline. Relative percent reductions comparing TARPEYO and placebo are estimated from the regression model.
Abbreviations: UPCR, urine protein to creatinine ratio; CI, confidence interval; LS, least squares.
In the final analysis of 364 patients, the trial met the prespecified Part B primary endpoint (p<0.0001). The mean change from baseline in eGFR and respective 95% CI for each arm at each scheduled visit during the treatment and follow-up phase is shown in Figure 2. The favorable effect of TARPEYO on eGFR was seen by Month 3 (the earliest assessment) and did not appear to increase in magnitude over two years. At Year 2, there was a 5.9 mL/min/1.73 m2 difference in the mean change from baseline in eGFR between TARPEYO and placebo (95% CI: 3.3 to 8.5 mL/min/1.73 m2; p<0.0001).
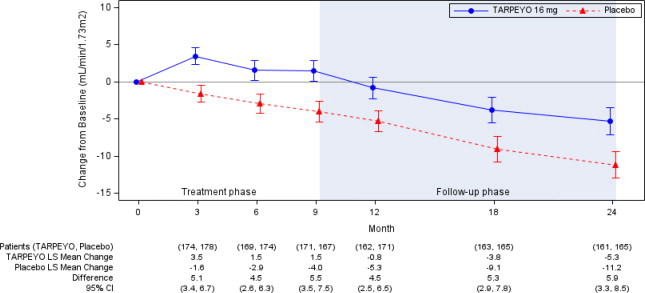
Estimated least squares mean change from baseline in eGFR (mL/min/1.73 m2) with 95% confidence intervals estimated from a mixed model repeated measures analysis of post-baseline to baseline differences at 3, 6, 9, 12, 18, and 24 months. Analysis was based on untransformed data and includes all eGFR data regardless of use of prohibited medication at any point during the study. A total of 15 patients in the TARPEYO arm and 20 patients in the placebo arm received rescue medication during the 2-year study.
Abbreviations: eGFR, estimated glomerular filtration rate; CI, confidence interval; LS least squares
The treatment effect based on the change from baseline in eGFR at 2 years was consistent across key subgroups, including key demographic (such as age, sex, race) and baseline disease (such as baseline proteinuria) characteristics.


When corticosteroids are used chronically, systemic effects such as hypercorticism and adrenal suppression may occur. Corticosteroids can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to surgery or other stress situations, supplementation with a systemic corticosteroid is recommended. When discontinuing therapy
Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure of oral budesonide. Avoid use in patients with severe hepatic impairment (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism in patients with moderate hepatic impairment (Child-Pugh Class B)
The delayed release capsules should be swallowed whole in the morning, at least 1 hour before a meal. Do not open, crush or chew.
If a dose is missed, take the prescribed dose at the next scheduled time. Do not double the next dose.
Safety and efficacy of treatment with subsequent courses of TARPEYO have not been established.
Delayed release capsule containing 4 mg budesonide. White coated opaque capsules printed with “CAL10 4MG” in black ink.
Lactation: Routine monitoring of linear growth in infants is recommended with chronic use of budesonide in the nursing mother. (
Breastfeeding is not expected to result in significant exposure of the infant to TARPEYO. Lactation studies have not been conducted with oral budesonide, including TARPEYO, and no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. One published study reports that budesonide is present in human milk following maternal inhalation of budesonide
One published study reports that budesonide is present in human milk following maternal inhalation of budesonide, which resulted in infant doses approximately 0.3% to 1% of the maternal weight-adjusted dosage and a milk to plasma ratio was approximately 0.5. Budesonide was not detected in plasma, and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide.
Assuming a daily average milk intake of about 150 mL/kg/day and a milk to plasma ratio of 0.5, the estimated oral dose of budesonide for a 5 kg infant is expected to be less than 2 mcg/day for a maternal dose of 16 mg TARPEYO. Assuming 100% bio-availability in the infant this is about 0.1% of the maternal dose and about 3% of the highest inhaled dose used clinically for asthma in infants.
TARPEYO is contraindicated in patients with hypersensitivity to budesonide or any of the ingredients of TARPEYO. Serious hypersensitivity reactions, including anaphylaxis have occurred with other budesonide formulations.
- Hypercorticism and Adrenal Axis Suppression:Follow general warnings concerning corticosteroids, patients with hepatic impairment may be at increased risk. Taper upon discontinuation. (,
2 DOSAGE AND ADMINISTRATIONThe recommended duration of therapy is 9 months, with a dosage of 16 mg administered orally once daily
[see Clinical Studies ]. When discontinuing therapy, reduce the dosage to 8 mg once daily for the last 2 weeks of therapy[see Warnings and Precautions ].The delayed release capsules should be swallowed whole in the morning, at least 1 hour before a meal. Do not open, crush or chew.
If a dose is missed, take the prescribed dose at the next scheduled time. Do not double the next dose.
Safety and efficacy of treatment with subsequent courses of TARPEYO have not been established.
- The recommended dosage is 16 mg administered orally once daily, in the morning at least 1 hour before a meal.
- Swallow whole. Do not open, crush or chew.
When discontinuing, reduce dosage to 8 mg once daily for the last two weeks.
,5.1 Hypercorticism and Adrenal Axis SuppressionWhen corticosteroids are used chronically, systemic effects such as hypercorticism and adrenal suppression may occur. Corticosteroids can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to surgery or other stress situations, supplementation with a systemic corticosteroid is recommended. When discontinuing therapy
[see Dosing and Administration ]or switching between corticosteroids, monitor for signs of adrenal axis suppression.Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure of oral budesonide. Avoid use in patients with severe hepatic impairment (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism in patients with moderate hepatic impairment (Child-Pugh Class B)
[see Use in Specific Populations , Clinical Pharmacology ].,8.6 Hepatic ImpairmentPatients with moderate to severe hepatic impairment (Child-Pugh Class B and C, respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure to budesonide
[see Warnings and Precautions and Clinical Pharmacology ]. Avoid use in patients with severe hepatic impairments (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism in patients with moderate hepatic impairment (Child-Pugh Class B).)12.3 PharmacokineticsAbsorptionFollowing single oral administration of TARPEYO 16 mg to healthy subjects, the average geometric mean Cmax(CV%) was 4.4 ng/mL (58.3), and AUC0-24was 24.1 h*ng/mL (49.7). Median Tlag(min, max) was 3.1 h (0, 6) while median Tmax(min, max) was 5.1 h (4.5, 10).
Food EffectThere was no clinically relevant food effect observed on the overall systemic exposure of budesonide when either a moderate or high fat meal was consumed 1 hour after administration of TARPEYO.
DistributionApproximately 85 to 90% of budesonide binds to plasma proteins in blood over the concentration range of 0.43 to 99 ng/mL. The volume of distribution at steady state reported in the literature is 3 to 4 L/kg.
MetabolismBudesonide is metabolized by the liver (and to lesser extent the gut), primarily by oxidative pathways via CYP3A4 to two main metabolites, 16α-hydroxyprednisolone and 6β-hydroxybudesonide, which have less than 1% of the corticosteroid activity of budesonide.
EliminationBudesonide had a high plasma clearance, 0.9 to 1.8 L/min in healthy adults, which is close to the estimated liver blood flow, and, accordingly, suggests that budesonide is a high hepatic clearance drug.
Following single oral administration of TARPEYO 16 mg to healthy subjects, the elimination half-life (t½) for TARPEYO ranged from 5.0 to 6.8 hours.
ExcretionBudesonide was excreted in urine and feces in the form of metabolites. After oral as well as intravenous administration of micronized [3H]-budesonide, approximately 60% of the recovered radioactivity was found in urine. The major metabolites, including 16α-hydroxyprednisolone and 6β-hydroxybudesonide, are mainly renally excreted, intact or in conjugated forms. No unchanged budesonide was detected in urine.
Specific PopulationsAge, race, and body weightThe effect of age, race, and body weight on the pharmacokinetics of TARPEYO has not been established.
SexOf the 143 healthy volunteers included in the Phase 1 studies, 29% were female. Pharmacokinetics of budesonide was similar between males and females.
Hepatic ImpairmentSubjects with moderate hepatic impairment (Child-Pugh class B) had 3.5 times the budesonide AUC compared with healthy volunteers while subjects with mild hepatic impairment (Child-Pugh class A) had approximately 1.4 times the budesonide AUC compared with healthy volunteers.
Patients with severe hepatic impairment (Child-Pugh Class C) have not been studied.
Renal ImpairmentIntact budesonide is not excreted renally. The main metabolites of budesonide, which have negligible corticosteroid activity, are largely (60%) excreted in urine.
Drug Interaction StudiesBudesonide is metabolized via CYP3A4. Potent inhibitors of CYP3A4 can increase plasma levels of budesonide.
Thus, clinically relevant drug interactions with potent CYP3A4 inhibitors, such as ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, cyclosporine, and grapefruit juice, are to be expected. Conversely, induction of CYP3A4 potentially could result in the lowering of budesonide plasma concentrations.
Effects of Other Drugs on BudesonideKetoconazoleIn an open, non-randomized, cross-over study, 6 healthy subjects were given budesonide 10 mg as a single dose, either alone or concomitantly with the last ketoconazole dose of 3 days treatment with ketoconazole 100 mg twice daily. Co-administration of ketoconazole resulted in 8-fold the AUC of budesonide, compared to budesonide alone.
In an open, randomized, cross-over study 8 healthy subjects were given Entocort EC 3 mg as a single dose, either alone or concomitantly with the last ketoconazole dose of 4 days treatment with ketoconazole 200 mg once daily. Co-administration of ketoconazole resulted in 6.5-fold the AUC of budesonide, compared to budesonide alone.
Grapefruit JuiceIn an open, randomized, cross-over study, 8 healthy subjects were given Entocort EC 3 mg, either alone, or concomitantly with 600 mL concentrated grapefruit juice (which inhibits CYP3A4 activity predominantly in the intestinal mucosa), on the last of 4 daily administrations. Concomitant administration of grapefruit juice resulted in doubling the bioavailability of budesonide compared to budesonide alone.
Proton Pump InhibitorsThe pharmacokinetics of TARPEYO have not been evaluated in combination with proton pump inhibitors (PPIs). Since the disintegration of TARPEYO is pH dependent, the release properties and uptake of budesonide may be altered when TARPEYO is taken after treatment with PPIs. In a study assessing intragastric and intraduodenal pH in healthy volunteers after repeated dosing with the PPI omeprazole 40 mg once daily, intragastric and intraduodenal pH did not exceed that required for disintegration of TARPEYO. Beyond the duodenum, PPIs such as omeprazole are unlikely to affect pH.
Oral Contraceptives (CYP3A4 Substrates)In a parallel study, the pharmacokinetics of budesonide were not significantly different between healthy female subjects who received oral contraceptives containing desogestrel 0.15 mg and ethinyl estradiol 30 μg and healthy female subjects who did not receive oral contraceptives. Budesonide 4.5 mg once daily for one week did not affect the plasma concentrations of ethinyl estradiol, a CYP3A4 substrate. The effect of budesonide 16 mg once daily on the plasma concentrations of desogestrel and ethinyl estradiol was not studied.
- Immunosuppression and Increased Risk of Infection:Avoid use in patients with active or quiescent tuberculosis infection, untreated fungal, bacterial, systemic viral or parasitic infections, or ocular herpes simplex. May affect vaccine efficacy. ()
5.2 Immunosuppression and Increased Risk of InfectionCorticosteroids, including TARPEYO, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
- Reduce resistance to new infections
- Exacerbate existing infections
- Increase the risk of disseminated infections
- Increase the risk of reactivation or exacerbation of latent infections
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider TARPEYO withdrawal as needed.
Tuberculosis
If TARPEYO is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. In patients with latent tuberculosis or tuberculin reactivity TARPEYO should be discontinued.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including TARPEYO. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- If a TARPEYO-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If a TARPEYO-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including TARPEYO. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive treatment with TARPEYO. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including TARPEYO, may exacerbate systemic fungal infections; therefore, avoid TARPEYO use in the presence of such infections.
Amebiasis
Corticosteroids, including TARPEYO, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating TARPEYO in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including TARPEYO, should be discontinued in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Cerebral Malaria
Avoid corticosteroids, including TARPEYO, in patients with cerebral malaria.
Ocular Herpes Simplex Virus Infection
Corticosteroids, including TARPEYO, may exacerbate ocular herpes simplex virus infections; therefore, avoid TARPEYO use in the presence of such infections.
Kaposi's Sarcoma
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi's sarcoma.
Immunizations
Corticosteroid therapy, including TARPEYO, may decrease the immune response to some vaccines.
- Other Corticosteroid Effects:Monitor patients with concomitant conditions where corticosteroids may have unwanted effects (e.g., hypertension, diabetes mellitus). ()
5.3 Other Corticosteroid EffectsTARPEYO is a systemically available corticosteroid and is expected to cause related adverse reactions. Monitor patients with hypertension, prediabetes, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma or cataracts, or with a family history of diabetes or glaucoma, or with any other condition where corticosteroids may have unwanted effects.