Tazarotene - Tazarotene cream
(Tazarotene)Tazarotene - Tazarotene cream Prescribing Information
Cream, 0.05% and 0.1%. Each gram of tazarotene cream, 0.05% and 0.1% contains 0.5 mg and 1 mg of tazarotene, respectively in a white to off-white cream base.
Tazarotene cream is contraindicated in:
- Pregnancy. Retinoids may cause fetal harm when administered to a pregnant female[see Warnings and Precautions (.), Use in Specific Populations (
5.1 Embryofetal ToxicitySystemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in orally treated animals. Although there may be less systemic exposure in the treatment of acne of the face alone due to less surface area for application, tazarotene is a teratogenic substance, and it is not known what level of exposure is required for teratogenicity in humans
[see Clinical Pharmacology ].There were thirteen reported pregnancies in subjects who participated in the clinical trials for topical tazarotene. Nine of the subjects were found to have been treated with topical tazarotene, and the other four had been treated with vehicle. One of the subjects who was treated with tazarotene cream elected to terminate the pregnancy for non-medical reasons unrelated to treatment. The other eight pregnant women who were inadvertently exposed to topical tazarotene during clinical trials subsequently delivered apparently healthy babies. As the exact timing and extent of exposure in relation to the gestation times are not certain, the significance of these findings is unknown.
Females of Child-bearing PotentialFemales of child-bearing potential should be warned of the potential risk and use adequate birth-control measures when tazarotene cream is used. The possibility that a female of child-bearing potential is pregnant at the time of institution of therapy should be considered.
A negative result for pregnancy test should be obtained within 2 weeks prior to tazarotene cream therapy. Tazarotene cream therapy should begin during a menstrual period
[see Use in Specific Populations ].,8.1 PregnancyRisk SummaryBased on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, tazarotene cream may cause fetal harm when administered to a pregnant female and is contraindicated during pregnancy. Safety in pregnant females has not been established. The potential risk to the fetus outweighs the potential benefit to the mother from tazarotene cream during pregnancy; therefore, tazarotene cream should be discontinued as soon as pregnancy is recognized
[see Contraindications , Warnings and Precautions , Clinical Pharmacology ].Limited case reports of pregnancy in females enrolled in clinical trials for tazarotene cream have not established a clear association with tazarotene and major birth defects or miscarriage risk. Because the exact timing and extent of exposure in relation to the gestational age are not certain, the significance of these findings is unknown.In animal reproduction studies with pregnant rats, tazarotene dosed topically during organogenesis at 2 times the maximum systemic exposure in subjects treated with the maximum recommended human dose (MRHD) of tazarotene cream, 0.1% resulted in reduced fetal body weights and reduced skeletal ossification. In animal reproduction studies with pregnant rabbits dosed topically with tazarotene gel at 26 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%, there was a single incident of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.
In animal reproduction studies with pregnant rats and rabbits, tazarotene dosed orally during organogenesis at 2 and 52 times, respectively, the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1% resulted in malformations, fetal toxicity, developmental delays, and/or behavioral delays
.In pregnant rats, tazarotene dosed orally prior to mating through early gestation resulted in decreased litter size, decreased numbers of live fetuses, decreased fetal body weights, and increased malformations at doses approximately 7 times higher than the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%[see Data].The background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
DataAnimal DataIn rats, a tazarotene gel, 0.05% formulation dosed topically during gestation days 6 through 17 at 0.25 mg/kg/day, which represented 2 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1% (i.e
., 2 mg/cm2over a 15% body surface area), resulted in reduced fetal body weights and reduced skeletal ossification. Rabbits dosed topically with 0.25 mg/kg/day tazarotene gel, which represented 26 times the maximum systemic exposure in subjects treated with MRHD of tazarotene cream, 0.1%, during gestation days 6 through 18, had a single incident of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies.When tazarotene was given orally to animals, developmental delays were seen in rats, and malformations and post-implantation loss were observed in rats and rabbits at doses representing 2 and 52 times, respectively, the maximum systemic exposure seen in subjects treated with the MRHD of tazarotene cream, 0.1%.
In female rats orally administered 2 mg/kg/day of tazarotene from 15 days before mating through gestation day 7, which represented 7 times the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%, classic developmental effects of retinoids were observed including decreased number of implantation sites, decreased litter size, decreased numbers of live fetuses, and decreased fetal body weights. A low incidence of retinoid-related malformations was observed at that dose.
In a pre-and postnatal development toxicity study, topical administration of tazarotene gel (0.125 mg/kg/day) to pregnant female rats from gestation day 16 through lactation day 20 reduced pup survival, but did not affect the reproductive capacity of the offspring. Based on data from another study, the maximum systemic exposure in the rat would be equivalent to the maximum systemic exposure in subjects treated with the MRHD of tazarotene cream, 0.1%.
)]8.3 Females and Males of Reproductive PotentialPregnancy TestingPregnancy testing is recommended for females of reproductive potential within 2 weeks prior to initiating tazarotene cream therapy which should begin during a menstrual period.
ContraceptionFemalesBased on animal studies, tazarotene cream may cause fetal harm when administered to a pregnant woman
[see Use in Specific Populations ].Advise females of reproductive potential to use effective contraception during treatment with tazarotene cream. - Individuals who have known hypersensitivity to any of its components[see Warnings and Precautions (.)]
5.2 Local Irritation and Hypersensitivity ReactionsLocal tolerability reactions (including blistering and skin desquamation) and hypersensitivity adverse reactions (including urticaria) have been observed with topical tazarotene. Application of tazarotene cream may cause excessive irritation in the skin of certain sensitive individuals. Some individuals may experience excessive pruritus, burning, skin redness or peeling. If these effects occur, the medication should either be discontinued until the integrity of the skin is restored, or the dosing should be reduced to an interval the patient can tolerate. However, efficacy at reduced frequency of application has not been established. Alternatively, patients with psoriasis who are being treated with the 0.1% concentration can be switched to the lower concentration. Frequency of application should be closely monitored by careful observation of the clinical therapeutic response and skin tolerance. Therapy can be resumed, or the drug concentration or frequency of application can be increased as the patient becomes able to tolerate treatment.
Concomitant topical medications and cosmetics that have a strong drying effect should be avoided. It is also advisable to "rest" a patient's skin until the effects of such preparations subside before use of tazarotene cream is begun.
Tazarotene cream, should not be used on eczematous skin, as it may cause severe irritation.
Weather extremes, such as wind or cold, may be more irritating to patients using tazarotene cream.
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Embryofetal toxicity[see Warnings and Precautions ()]
5.1 Embryofetal ToxicitySystemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area, exposure could be in the same order of magnitude as in orally treated animals. Although there may be less systemic exposure in the treatment of acne of the face alone due to less surface area for application, tazarotene is a teratogenic substance, and it is not known what level of exposure is required for teratogenicity in humans
[see Clinical Pharmacology ].There were thirteen reported pregnancies in subjects who participated in the clinical trials for topical tazarotene. Nine of the subjects were found to have been treated with topical tazarotene, and the other four had been treated with vehicle. One of the subjects who was treated with tazarotene cream elected to terminate the pregnancy for non-medical reasons unrelated to treatment. The other eight pregnant women who were inadvertently exposed to topical tazarotene during clinical trials subsequently delivered apparently healthy babies. As the exact timing and extent of exposure in relation to the gestation times are not certain, the significance of these findings is unknown.
Females of Child-bearing PotentialFemales of child-bearing potential should be warned of the potential risk and use adequate birth-control measures when tazarotene cream is used. The possibility that a female of child-bearing potential is pregnant at the time of institution of therapy should be considered.
A negative result for pregnancy test should be obtained within 2 weeks prior to tazarotene cream therapy. Tazarotene cream therapy should begin during a menstrual period
[see Use in Specific Populations ]. - Photosensitivity and Risk of Sunburn[see Warnings and Precautions ()]
5.3 Photosensitivity and Risk for SunburnBecause of heightened burning susceptibility, exposure to sunlight (including sunlamps) should be avoided unless deemed medically necessary, and in such cases, exposure should be minimized during the use of tazarotene cream. Patients must be warned to use sunscreens and protective clothing when using tazarotene cream. Patients with sunburn should be advised not to use tazarotene cream until fully recovered. Patients who may have considerable sun exposure due to their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using tazarotene cream.
Tazarotene cream should be administered with caution if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
No formal drug-drug interaction studies were conducted with tazarotene cream.
In a trial of 27 healthy female subjects between the ages of 20-55 years receiving a combination oral contraceptive tablet containing 1 mg norethindrone and 35 mcg ethinyl estradiol, concomitant use of tazarotene administered as 1.1 mg orally (mean ± SD C
max and AUC
0-24 of tazarotenic acid were 28.9 ± 9.4 ng/mL and 120.6 ± 28.5 ng·hr/mL) did not affect the pharmacokinetics of norethindrone and ethinyl estradiol over a complete cycle.
The impact of tazarotene on the pharmacokinetics of progestin only oral contraceptives (i.e., minipills) has not been evaluated.
Tazarotene cream, 0.05% and 0.1% is for topical use and contains the active ingredient, tazarotene. Each gram of tazarotene cream, 0.05% and 0.1% contains 0.5 mg and 1 mg of tazarotene, respectively in a white to off-white cream base.
Tazarotene is a member of the acetylenic class of retinoids. Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl)ethynyl]nicotinate. The compound has an empirical formula of C
21H
21NO
2S and molecular weight of 351.46. The structural formula is shown below: 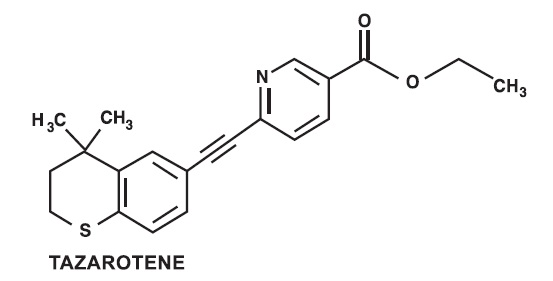
Tazarotene cream contains the following inactive ingredients: benzyl alcohol 1%; carbomer copolymer type A; carbomer homopolymer type B; edetate disodium; medium chain triglycerides; mineral oil; purified water; sodium hydroxide; sodium thiosulfate; and sorbitan monooleate.
In two 12-week vehicle-controlled clinical trials, tazarotene cream, 0.05% and 0.1% was significantly more effective than vehicle in reducing the severity of stable plaque psoriasis. Tazarotene cream 0.1% and 0.05% demonstrated superiority over vehicle cream as early as 1 week and 2 weeks, respectively, after starting treatment.
In these trials, the primary efficacy endpoint was “clinical success,” defined as the proportion of subjects with none, minimal, or mild overall lesional assessment at Week 12, and shown in
| Table 1. Subject Numbers and Percentages for Overall Lesional Assessment Scores and “Clinical Success” at Baseline (BL), End of Treatment (Week 12) and 12 Weeks After Stopping Therapy (Week 24)#in Two Controlled Clinical Trials for Psoriasis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
0 no plaque elevation above normal skin level; may have residual non-erythematous discoloration; no psoriatic scale Clinical Success defined as an overall lesional assessment score of none, minimal, or mild. # Trial 1 had post-treatment period observations for 12 weeks after stopping therapy, which were not part of Trial 2. * Denotes statistically significant difference for "Clinical Success" compared with vehicle. | |||||||||||||||
Tazarotene Cream, 0.05% | Tazarotene Cream, 0.1% | Vehicle Cream | |||||||||||||
Trial 1 N=218 | Trial 2 N=210 | Trial 1 N=221 | Trial 2 N=211 | Trial 1 N=229 | Trial 2 N=214 | ||||||||||
| Score | BL | Wk 12 | Wk 24 | BL | Wk 12 | BL | Wk 12 | Wk 24 | BL | Wk 12 | BL | Wk 12 | Wk 24 | BL | Wk 12 |
| None (0) | 0 | 1 (0.5%) | 1 (0.5%) | 0 | 2 (1%) | 0 | 0 | 0 | 0 | 6 (3%) | 0 | 0 | 1 (0.4%) | 0 | 1 (0.5%) |
| Minimal (1) | 0 | 11 (5%) | 12 (6%) | 0 | 7 (3%) | 0 | 12 (5%) | 14 (6%) | 0 | 11 (5%) | 0 | 7 (3%) | 6 (3%) | 0 | 1 (0.5%) |
| Mild (2) | 0 | 79 (36%) | 60 (28%) | 0 | 76 (36%) | 0 | 75 (34%) | 53 (24%) | 0 | 90 (43%) | 0 | 49 (21%) | 43 (19%) | 0 | 54 (25%) |
| Moderate (3) | 141 (65%) | 86 (39%) | 90 (41%) | 100 (48%) | 74 (35%) | 122 (55%) | 97 (44%) | 107 (48%) | 96 (45%) | 62 (29%) | 139 (61%) | 119 (52%) | 114 (50%) | 97 (45%) | 99 (46%) |
| Severe (4) | 69 (32%) | 39 (18%) | 51 (23%) | 80 (38%) | 36 (17%) | 91 (41%) | 36 (16%) | 46 (21%) | 86 (41%) | 29 (14%) | 81 (35%) | 51 (22%) | 61 (27%) | 93 (44%) | 47 (22%) |
| Very Severe (5) | 8 (4%) | 2 (0.9%) | 4 (2%) | 30 (14%) | 15 (7%) | 8 (4%) | 1 (0.5%) | 1 (0.5%) | 29 (14%) | 13 (6%) | 9 (4%) | 3 (1%) | 4 (2%) | 24 (11%) | 12 (6%) |
| "Clinical Success" | 0 | 91 (42%*) | 73 (33%*) | 0 | 85 (40%*) | 0 | 87 (39%*) | 67 (30%*) | 0 | 107 (51%*) | 0 | 56 (24%) | 50 (22%) | 0 | 56 (26%) |
| Table 1. Subject Numbers and Percentages for Overall Lesional Assessment Scores and “Clinical Success” at Baseline (BL), End of Treatment (Week 12) and 12 Weeks After Stopping Therapy (Week 24) # in Two Controlled Clinical Trials for Psoriasis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
0 no plaque elevation above normal skin level; may have residual non-erythematous discoloration; no psoriatic scale Clinical Success defined as an overall lesional assessment score of none, minimal, or mild. # Trial 1 had post-treatment period observations for 12 weeks after stopping therapy, which were not part of Trial 2. * Denotes statistically significant difference for "Clinical Success" compared with vehicle. | |||||||||||||||
Tazarotene Cream, 0.05% | Tazarotene Cream, 0.1% | Vehicle Cream | |||||||||||||
Trial 1 N=218 | Trial 2 N=210 | Trial 1 N=221 | Trial 2 N=211 | Trial 1 N=229 | Trial 2 N=214 | ||||||||||
| Score | BL | Wk 12 | Wk 24 | BL | Wk 12 | BL | Wk 12 | Wk 24 | BL | Wk 12 | BL | Wk 12 | Wk 24 | BL | Wk 12 |
| None (0) | 0 | 1 (0.5%) | 1 (0.5%) | 0 | 2 (1%) | 0 | 0 | 0 | 0 | 6 (3%) | 0 | 0 | 1 (0.4%) | 0 | 1 (0.5%) |
| Minimal (1) | 0 | 11 (5%) | 12 (6%) | 0 | 7 (3%) | 0 | 12 (5%) | 14 (6%) | 0 | 11 (5%) | 0 | 7 (3%) | 6 (3%) | 0 | 1 (0.5%) |
| Mild (2) | 0 | 79 (36%) | 60 (28%) | 0 | 76 (36%) | 0 | 75 (34%) | 53 (24%) | 0 | 90 (43%) | 0 | 49 (21%) | 43 (19%) | 0 | 54 (25%) |
| Moderate (3) | 141 (65%) | 86 (39%) | 90 (41%) | 100 (48%) | 74 (35%) | 122 (55%) | 97 (44%) | 107 (48%) | 96 (45%) | 62 (29%) | 139 (61%) | 119 (52%) | 114 (50%) | 97 (45%) | 99 (46%) |
| Severe (4) | 69 (32%) | 39 (18%) | 51 (23%) | 80 (38%) | 36 (17%) | 91 (41%) | 36 (16%) | 46 (21%) | 86 (41%) | 29 (14%) | 81 (35%) | 51 (22%) | 61 (27%) | 93 (44%) | 47 (22%) |
| Very Severe (5) | 8 (4%) | 2 (0.9%) | 4 (2%) | 30 (14%) | 15 (7%) | 8 (4%) | 1 (0.5%) | 1 (0.5%) | 29 (14%) | 13 (6%) | 9 (4%) | 3 (1%) | 4 (2%) | 24 (11%) | 12 (6%) |
| "Clinical Success" | 0 | 91 (42%*) | 73 (33%*) | 0 | 85 (40%*) | 0 | 87 (39%*) | 67 (30%*) | 0 | 107 (51%*) | 0 | 56 (24%) | 50 (22%) | 0 | 56 (26%) |
At the end of 12 weeks of treatment, tazarotene cream, 0.05% and 0.1% was consistently superior to vehicle in reducing the plaque thickness of psoriasis. Improvements in erythema and scaling were generally significantly greater with tazarotene cream, 0.05% and 0.1% than with vehicle. Tazarotene cream, 0.1% was also generally more effective than tazarotene cream, 0.05% in reducing the severity of the individual signs of disease. However, tazarotene cream, 0.1% was associated with a greater degree of local irritation than tazarotene cream, 0.05%
Table 2. Mean Decreases in Plaque Elevation, Scaling and Erythema in Two Controlled Clinical Trials for Psoriasis | |||||||||||||||||||
Tazarotene Cream, 0.05% | Tazarotene Cream, 0.1% | Vehicle Cream | |||||||||||||||||
Lesion | Trunk/Arm/ Leg lesions | Knee/Elbow lesions | All Treated | Trunk/Arm/ Leg lesions | Knee/Elbow lesions | All Treated | Trunk/Arm/ Leg lesions | Knee/Elbow lesions | All Treated | ||||||||||
Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | Trial 1 | Trial 2 | ||
N=218 | N=210 | N=218 | N=210 | N=218 | N=210 | N=221 | N=211 | N=221 | N=211 | N=221 | N=211 | N=229 | N=214 | N=229 | N=214 | N=229 | N=214 | ||
Plaque elevation | B# C-12 C-24 | 2.29 -0.83* -0.75* | 2.50 -0.98* | 2.40 -0.91* -0.73* | 2.52 -1.04* | 2.28 -0.75* -0.60* | 2.51 -0.90* | 2.34 -1.08* -0.87* | 2.52 -1.25* | 2.35 -0.96* -0.73* | 2.49 -1.21* | 2.32 -0.83* -0.63* | 2.51 -1.08* | 2.28 - 0.59-0.57 | 2.51 -0.69 | 2.35 - 0.57-0.49 | 2.51 -0.68 | 2.29 - 0.48-0.42 | 2.51 -0.61 |
Scaling | B# C-12 C-24 | 2.26 - 0.75-0.68 | 2.45 -0.90 | 2.47 -0.78* -0.62* | 2.60 -0.98* | 2.32 -0.67* -0.51* | 2.47 -0.80 | 2.37 -0.84* -0.79* | 2.45 -1.06* | 2.40 -0.76* -0.61* | 2.57 -1.13* | 2.36 -0.73* -0.59* | 2.53 -1.03* | 2.34 - 0.66-0.56 | 2.46 -0.79 | 2.45 - 0.62-0.45 | 2.61 -0.76 | 2.31 - 0.46-0.34 | 2.53 -0.70 |
Erythema | B# C-12 C-24 | 2.26 - 0.49-0.52 | 2.51 -0.65* | 2.17 - 0.44-0.44 | 2.40 -0.66* | 2.23 - 0.40-0.41 | 2.48 -0.62 | 2.25 - 0.49-0.55 | 2.53 -0.82* | 2.17 -0.57* -0.52* | 2.42 -0.82* | 2.21 -0.42* -0.39* | 2.51 -0.78* | 2.24 - 0.42-0.43 | 2.47 -0.46 | 2.17 - 0.38-0.34 | 2.34 -0.44 | 2.24 - 0.37-0.33 | 2.47 -0.47 |
Plaque elevation, scaling and erythema scored on a 0-4 scale with 0=none, 1=mild, 2=moderate, 3=severe and 4=very severe. B#=Mean Baseline Severity; * Denotes statistically significant difference compared with vehicle. | |||||||||||||||||||
In two large vehicle-controlled trials, subjects age 12 years and over with facial acne vulgaris of a severity suitable for monotherapy with a topical agent were enrolled. After face cleansing in the evening, tazarotene cream, 0.1% was applied once daily to the entire face as a thin layer. Tazarotene cream, 0.1% was significantly more effective than vehicle in the treatment of facial acne vulgaris. Efficacy results after 12 weeks of treatment are shown in
| Table 3. Efficacy Results after Twelve Weeks of Treatment in Two Controlled Clinical Trials for Acne | ||||
|---|---|---|---|---|
* Denotes statistically significant difference compared with vehicle. | ||||
Tazarotene Cream, 0.1% | Vehicle Cream | |||
Trial 1 N=218 | Trial 2 N=206 | Trial 1 N=218 | Trial 2 N=205 | |
| Median Percent Reduction in ● Noninflammatory lesions ● Inflammatory lesions ● Total lesions | 46%* 41%* 44%* | 41%* 44%* 42%* | 27% 27% 24% | 21% 25% 21% |
| Percent of Subjects with No Acne or Minimal Acne | 18%* | 20%* | 11% | 6% |
| Percent of Subjects with No Acne, Minimal Acne, or Mild Acne | 55%* | 53%* | 36% | 36% |
| Table 3. Efficacy Results after Twelve Weeks of Treatment in Two Controlled Clinical Trials for Acne | ||||
|---|---|---|---|---|
* Denotes statistically significant difference compared with vehicle. | ||||
Tazarotene Cream, 0.1% | Vehicle Cream | |||
Trial 1 N=218 | Trial 2 N=206 | Trial 1 N=218 | Trial 2 N=205 | |
| Median Percent Reduction in ● Noninflammatory lesions ● Inflammatory lesions ● Total lesions | 46%* 41%* 44%* | 41%* 44%* 42%* | 27% 27% 24% | 21% 25% 21% |
| Percent of Subjects with No Acne or Minimal Acne | 18%* | 20%* | 11% | 6% |
| Percent of Subjects with No Acne, Minimal Acne, or Mild Acne | 55%* | 53%* | 36% | 36% |