Technetium Tc 99m Sestamibi
Technetium Tc 99m Sestamibi Prescribing Information
Myocardial Imaging: Kit for the Preparation of Technetium Tc99m Sestamibi Injection is a myocardial perfusion agent that is indicated for detecting coronary artery disease by localizing myocardial ischemia (reversible defects) and infarction (non-reversible defects), in evaluating myocardial function and developing information for use in patient management decisions. Technetium Tc99m Sestamibi evaluation of myocardial ischemia can be accomplished with rest and cardiovascular stress techniques (e.g., exercise or pharmacologic stress in accordance with the pharmacologic stress agent's labeling).
It is usually not possible to determine the age of a myocardial infarction or to differentiate a recent myocardial infarction from ischemia.
Breast Imaging: Kit for the Preparation of Technetium Tc99m Sestamibi Injection is indicated for planar imaging as a second line diagnostic drug after mammography to assist in the evaluation of breast lesions in patients with an abnormal mammogram or a palpable breast mass.
Kit for the Preparation of Technetium Tc99m Sestamibi Injection is not indicated for breast cancer screening, to confirm the presence or absence of malignancy, and it is not an alternative to biopsy.
For Myocardial Imaging: The suggested dose range for I.V. administration of Technetium Tc99m Sestamibi in a single dose to be employed in the average patient (70 Kg) is 370–1110 MBq (10–30 mCi).
For Breast Imaging: The recommended dose range for I.V. administration of Technetium Tc99m Sestamibi is a single dose of 740–1110 MBq (20–30 mCi).
Kit for the Preparation of Technetium Tc99m Sestamibi Injection is supplied as a lyophilized mixture in a 10 mL vial.
None known.
Adverse events were evaluated in 3741 adults who were evaluated in clinical studies. Of these patients, 3068 (77% men, 22% women, and 0.7% of the patients' genders were not recorded) were in cardiac clinical trials and 673 (100% women) in breast imaging trials. Cases of angina, chest pain, and death have occurred (see
- Pharmacologic induction of cardiovascular stress may be associated with serious adverse events such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction and cerebrovascular events.
- Technetium Tc99m Sestamibi has been rarely associated with acute severe allergic and anaphylactic events of angioedema and generalized urticaria. In some patients the allergic symptoms developed on the second injection during Technetium Tc99m Sestamibi imaging.
- Caution should be exercised and emergency equipment should be available when administering Technetium Tc99m Sestamibi.
- Before administering Technetium Tc99m Sestamibi patients should be asked about the possibility of allergic reactions to either drug.
- The contents of the vial are intended only for use in the preparation of Technetium Tc99m Sestamibi and are not to be administered directly to the patient without first undergoing the preparative procedure.
In studying patients in whom cardiac disease is known or suspected, care should be taken to assure continuous monitoring and treatment in accordance with safe, accepted clinical procedure. Infrequently, death has occurred 4 to 24 hours after Tc99m Sestamibi use and is usually associated with exercise stress testing (See Section 5.2).
Pharmacologic induction of cardiovascular stress may be associated with serious adverse events such as myocardial infarction, arrhythmia, hypotension, bronchoconstriction and cerebrovascular events. Caution should be used when pharmacologic stress is selected as an alternative to exercise; it should be used when indicated and in accordance with the pharmacologic stress agent's labeling.
Technetium Tc99m Sestamibi has been rarely associated with acute severe allergic and anaphylactic events of angioedema and generalized urticaria. In some patients the allergic symptoms developed on the second injection during Tc99m Sestamibi imaging. Patients who receive Technetium Tc99m Sestamibi for either myocardial or breast imaging are receiving the same drug. Caution should be exercised and emergency equipment should be available when administering Technetium Tc99m Sestamibi. Also, before administering Technetium Tc99m Sestamibi Injection, patients should be asked about the possibility of allergic reactions to the drug.
The contents of the vial are intended only for use in the preparation of Technetium Tc99m Sestamibi and are not to be administered directly to the patient without first undergoing the preparative procedure.
Radioactive drugs must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Also, care should be taken to minimize radiation exposure to the patients consistent with proper patient management.
Contents of the kit before preparation are not radioactive. However, after the Sodium Pertechnetate Tc99m Injection is added, adequate shielding of the final preparation must be maintained. The components of the kit are sterile and non-pyrogenic. It is essential to follow directions carefully and to adhere to strict aseptic procedures during preparation.
Technetium Tc99m labeling reactions depend on maintaining the stannous ion in the reduced state. Hence, Sodium Pertechnetate Tc99m Injection containing oxidants should not be used.
Technetium Tc99m Sestamibi should not be used more than six hours after preparation.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides.
Stress testing should be performed only under the supervision of a qualified physician and in a laboratory equipped with appropriate resuscitation and support apparatus.
The most frequent exercise stress test endpoints sufficient to stop the test reported during controlled studies (two-thirds were cardiac patients) were:
Fatigue 35%
Dyspnea 17%
Chest Pain 16%
ST-depression 7%
Arrhythmia 1%
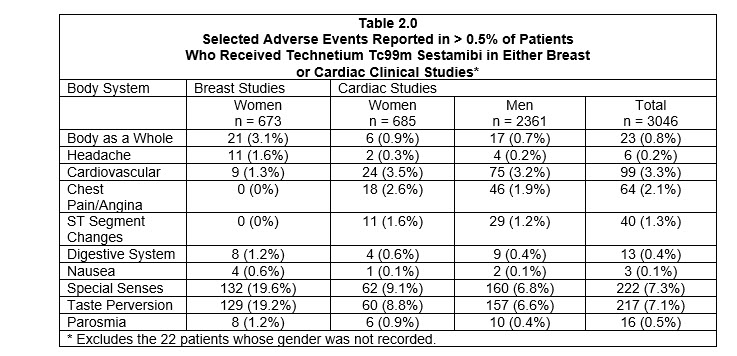
In the clinical studies for breast imaging, breast pain was reported in 12 (1.7%) of the patients. In 11 of these patients the pain appears to be associated with biopsy/surgical procedures.
The following adverse reactions have been reported in ≤ 0.5% of patients: signs and symptoms consistent with seizure occurring shortly after administration of the agent; transient arthritis; angioedema, arrhythmia, dizziness, syncope, abdominal pain, vomiting, and severe hypersensitivity characterized by dyspnea, hypotension, bradycardia, asthenia, and vomiting within two hours after a second injection of Technetium Tc99m Sestamibi. A few cases of flushing, edema, injection site inflammation, dry mouth, fever, pruritus, rash, urticaria and fatigue have also been attributed to administration of the agent.
Specific drug-drug interactions have not been studied.