Temozolomide
Temozolomide Prescribing Information
Indications and Usage (
- adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma;
- treatment of adults with refractory anaplastic astrocytoma.
Dosage and Administration (
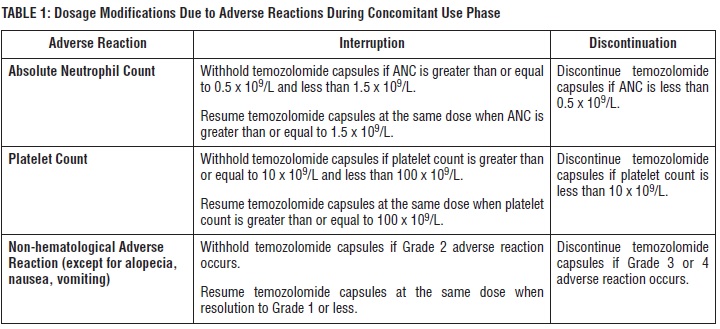
Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
If temozolomide capsules are withheld, reduce the dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day.
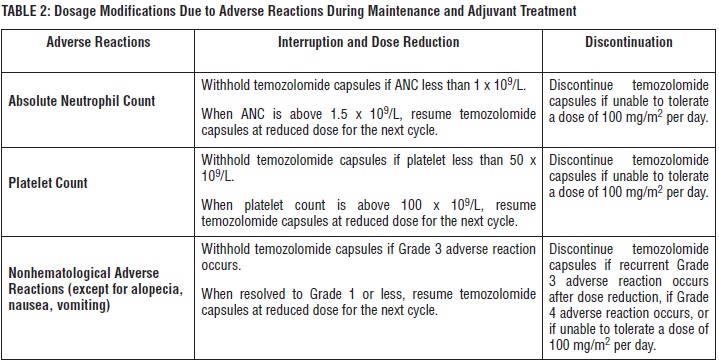


The recommended initial dosage of temozolomide capsules is 150 mg/m2once daily on Days 1 to 5 of each 28-day cycle. Increase the temozolomide dose to 200 mg/m2per day if the following conditions are met at the nadir and on Day 1 of the next cycle:
• ANC is greater than or equal to 1.5 x 109/L, and
• Platelet count is greater than or equal to 100 x 109/L.
If the ANC is less than 1 x 109/L or the platelet count is less than 50 x 109/L during any cycle, reduce the temozolomide dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day.
Take temozolomide capsules at the same time each day. Administer temozolomide capsules consistently with respect to food (fasting vs. nonfasting) [
If capsules are accidentally opened or damaged, take precautions to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, wash the affected area with water immediately.
Contraindications (
- temozolomide or any other ingredients in temozolomide capsules; and
- dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
Reactions to temozolomide have included anaphylaxis
- History of serious hypersensitivity to temozolomide or any other ingredients in temozolomide capsules and dacarbazine.
Warnings and Precautions (
Myelosuppression, including pancytopenia, leukopenia, and anemia, some with fatal outcomes, have occurred with temozolomide
Based on findings from animal studies and its mechanism of action, temozolomide can cause fetal harm when administered to a pregnant woman. Adverse developmental outcomes have been reported in both pregnant patients and pregnant partners of male patients. Oral administration of temozolomide to rats and rabbits during the period of organogenesis resulted in embryolethality and polymalformations at doses less than the maximum human dose based on body surface area.
Temozolomide is an alkylating drug indicated for the treatment of adults with:
- Newly diagnosed glioblastoma concomitantly with radiotherapy and then as maintenance treatment. ()
1.1 Newly Diagnosed GlioblastomaTemozolomide capsules are indicated for the treatment of adults with newly diagnosed glioblastoma, concomitantly with radiotherapy and then as maintenance treatment.
- Anaplastic astrocytoma. ()
1.2 Anaplastic AstrocytomaTemozolomide capsules are indicated for the:- adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma;
- treatment of adults with refractory anaplastic astrocytoma.
- Adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma. ()
1.2 Anaplastic AstrocytomaTemozolomide capsules are indicated for the:- adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma;
- treatment of adults with refractory anaplastic astrocytoma.
- Treatment of adults with refractory anaplastic astrocytoma. ()
1.2 Anaplastic AstrocytomaTemozolomide capsules are indicated for the:- adjuvant treatment of adults with newly diagnosed anaplastic astrocytoma;
- treatment of adults with refractory anaplastic astrocytoma.
- Administer orally. ()
2.4 Preparation and AdministrationTemozolomide is a hazardous drug. Follow applicable special handling and disposal procedures.1Temozolomide capsulesTake temozolomide capsules at the same time each day. Administer temozolomide capsules consistently with respect to food (fasting vs. nonfasting) [
see Clinical Pharmacology (12.3)]. To reduce nausea and vomiting, take temozolomide capsules on an empty stomach or at bedtime and consider antiemetic therapy prior to and following temozolomide capsule administration.Swallow temozolomide capsules whole with water. Advise patients not to open, chew, or dissolve the contents of the capsules [see Warnings and Precautions (5.6)].If capsules are accidentally opened or damaged, take precautions to avoid inhalation or contact with the skin or mucous membranes. In case of powder contact, wash the affected area with water immediately.
- Newly Diagnosed Glioblastoma:
- 75 mg/m2 once daily for 42 to 49 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1 to 5 of each 28-day cycle for 6 cycles. May increase maintenance dose to 200 mg/m2 for Cycles 2 to 6 based on toxicity. ()
2.2 Recommended Dosage and Dosage Modifications for Newly Diagnosed GlioblastomaAdminister temozolomide capsules once daily for 42 to 49 consecutive days during the concomitant use phase with focal radiotherapy, and then once daily on Days 1 to 5 of each 28-day cycle for 6 cycles during the maintenance use phase.ProvidePneumocystispneumonia (PCP) prophylaxis during the concomitant use phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less[see Warnings and Precautions (5.3)].Concomitant Use Phase:The recommended dosage of temozolomide capsules is 75 mg/m2once daily for 42 to 49 days in combination with focal radiotherapy. Focal radiotherapy includes the tumor bed or resection site with a 2 to 3 cm margin.Other administration schedules have been used.Obtain a complete blood count weekly. The recommended dosage modifications due to adverse reactions during concomitant use phase are provided inTable 1. Single Agent Maintenance Use Phase:Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is:•Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 6: May increase to 200 mg/m2per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2,do notincrease the dose for Cycles 3 to 6.
Single Agent Maintenance Use Phase:Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is:•Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 6: May increase to 200 mg/m2per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2,do notincrease the dose for Cycles 3 to 6.Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
The recommended dosage modifications due to adverse reactions during the maintenance use phase are provided inTable 2.If temozolomide capsules are withheld, reduce the dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day.


temozolomide-table1 
temozolomide-table2 - ProvidePneumocystispneumonia (PCP) prophylaxis during concomitant phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less. ()
2.2 Recommended Dosage and Dosage Modifications for Newly Diagnosed GlioblastomaAdminister temozolomide capsules once daily for 42 to 49 consecutive days during the concomitant use phase with focal radiotherapy, and then once daily on Days 1 to 5 of each 28-day cycle for 6 cycles during the maintenance use phase.ProvidePneumocystispneumonia (PCP) prophylaxis during the concomitant use phase and continue in patients who develop lymphopenia until resolution to Grade 1 or less[see Warnings and Precautions (5.3)].Concomitant Use Phase:The recommended dosage of temozolomide capsules is 75 mg/m2once daily for 42 to 49 days in combination with focal radiotherapy. Focal radiotherapy includes the tumor bed or resection site with a 2 to 3 cm margin.Other administration schedules have been used.Obtain a complete blood count weekly. The recommended dosage modifications due to adverse reactions during concomitant use phase are provided inTable 1. Single Agent Maintenance Use Phase:Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is:•Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 6: May increase to 200 mg/m2per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2,do notincrease the dose for Cycles 3 to 6.
Single Agent Maintenance Use Phase:Beginning 4 weeks after concomitant use phase completion, administer temozolomide capsules once daily on Days 1 to 5 of each 28-day cycle for 6 cycles. The recommended dosage of temozolomide capsules in the maintenance use phase is:•Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 6: May increase to 200 mg/m2per day on days 1 to 5 before starting Cycle 2 if no dosage interruptions or discontinuations are required (Table 1). If the dose is not escalated at the onset of Cycle 2,do notincrease the dose for Cycles 3 to 6.Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
The recommended dosage modifications due to adverse reactions during the maintenance use phase are provided inTable 2.If temozolomide capsules are withheld, reduce the dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day.


temozolomide-table1 
temozolomide-table2
- 75 mg/m2 once daily for 42 to 49 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1 to 5 of each 28-day cycle for 6 cycles. May increase maintenance dose to 200 mg/m2 for Cycles 2 to 6 based on toxicity. (
- Adjuvant Treatment of Newly Diagnosed Anaplastic Astrocytoma: Beginning 4 weeks after the end of radiotherapy, administer temozolomide orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage for Cycle 1 is 150 mg/m2 per day and for Cycles 2 to 12 is 200 mg/m2 if patient experienced no or minimal toxicity in Cycle 1. ()
2.3 Recommended Dosage and Dosage Modifications for Anaplastic AstrocytomaAdjuvant Treatment of Newly Diagnosed Anaplastic AstrocytomaBeginning 4 weeks after the end of radiotherapy, administer temozolomide capsules orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage of temozolomide capsules is:• Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 12: 200 mg/m2per day on days 1 to 5 if patient experienced no or minimal toxicity in Cycle 1. If the dose was not escalated at the onset of Cycle 2,do notincrease the dose during Cycles 3 to 6.The recommended complete blood count testing and dosage modifications due to adverse reactions during adjuvant treatment are provided above and in Table 2[see Dosage and Administration (2.2)].Refractory Anaplastic Astrocytoma
The recommended initial dosage of temozolomide capsules is 150 mg/m2once daily on Days 1 to 5 of each 28-day cycle. Increase the temozolomide dose to 200 mg/m2per day if the following conditions are met at the nadir and on Day 1 of the next cycle:
• ANC is greater than or equal to 1.5 x 109/L, and
• Platelet count is greater than or equal to 100 x 109/L.Continue temozolomide capsules until disease progression or unacceptable toxicity.Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
If the ANC is less than 1 x 109/L or the platelet count is less than 50 x 109/L during any cycle, reduce the temozolomide dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day. - Refractory Anaplastic Astrocytoma:Initial dose of 150 mg/m2 once daily on Days 1 to 5 of each 28-day cycle. ()
2.3 Recommended Dosage and Dosage Modifications for Anaplastic AstrocytomaAdjuvant Treatment of Newly Diagnosed Anaplastic AstrocytomaBeginning 4 weeks after the end of radiotherapy, administer temozolomide capsules orally in a single dose on days 1 to 5 of a 28-day cycle for 12 cycles. The recommended dosage of temozolomide capsules is:• Cycle 1: 150 mg/m2per day on days 1 to 5.• Cycles 2 to 12: 200 mg/m2per day on days 1 to 5 if patient experienced no or minimal toxicity in Cycle 1. If the dose was not escalated at the onset of Cycle 2,do notincrease the dose during Cycles 3 to 6.The recommended complete blood count testing and dosage modifications due to adverse reactions during adjuvant treatment are provided above and in Table 2[see Dosage and Administration (2.2)].Refractory Anaplastic Astrocytoma
The recommended initial dosage of temozolomide capsules is 150 mg/m2once daily on Days 1 to 5 of each 28-day cycle. Increase the temozolomide dose to 200 mg/m2per day if the following conditions are met at the nadir and on Day 1 of the next cycle:
• ANC is greater than or equal to 1.5 x 109/L, and
• Platelet count is greater than or equal to 100 x 109/L.Continue temozolomide capsules until disease progression or unacceptable toxicity.Obtain a complete blood count on Day 22 and then weekly until the ANC is above 1.5 x 109/L and the platelet count is above 100 x 109/L. Do not start the next cycle until the ANC and platelet count exceed these levels.
If the ANC is less than 1 x 109/L or the platelet count is less than 50 x 109/L during any cycle, reduce the temozolomide dose for the next cycle by 50 mg/m2per day. Permanently discontinue temozolomide capsules in patients who are unable to tolerate a dose of 100 mg/m2per day.
- Temozolomide capsules, USP for oral administration
-5 mg capsules have opaque white bodies with opaque light green caps. The capsule body is printed with "604" in black ink and the cap is printed with "LP" in black ink.
-20 mg capsules have opaque white bodies with opaque yellow caps. The capsule body is printed with "605" in black ink and the cap is printed with "LP" in black ink.
-100 mg capsules have opaque white bodies with opaque pink caps. The capsule body is printed with "606" in black ink and the cap is printed with "LP" in black ink.
-140 mg capsules have opaque white bodies with opaque blue caps. The capsule body is printed with "607" in black ink and the cap is printed with "LP" in black ink.
-180 mg capsules have opaque white bodies with opaque swedish orange caps. The capsule body is printed with "608" in black ink and the cap is printed with "LP" in black ink.
-250 mg capsules have opaque white bodies with opaque white caps. The capsule body is printed with "609" in black ink and the cap is printed with "LP" in black ink.
- Lactation: Advise not to breastfeed. ()
8.2 LactationThere are no data on the presence of temozolomide or its metabolites in human milk, the effects on a breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions, including myelosuppression from temozolomide in the breastfed children, advise women not to breastfeed during treatment with temozolomide and for 1 week after the last dose.
- temozolomide or any other ingredients in temozolomide capsules; and
- dacarbazine, since both temozolomide and dacarbazine are metabolized to the same active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide.
Reactions to temozolomide have included anaphylaxis
The following adverse reactions have been identified during post-approval use of temozolomide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.