Terbutaline Sulfate Prescribing Information
WARNING: PROLONGED TOCOLYSIS
Terbutaline Sulfate has not been approved for and should not be used for prolonged tocolysis (beyond 48-72 hours). In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration. [See CONTRAINDICATIONS, Prolonged Tocolysis.]
Terbutaline sulfate injection is indicated for the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema.
Vials should be used only for subcutaneous administration and not intravenous infusion. Sterility and accurate dosing cannot be assured if the vials are not used in accordance with DOSAGE AND ADMINISTRATION.
Discard unused portion after single patient use.
The usual subcutaneous dose of terbutaline sulfate injection is 0.25 mg injected into the lateral deltoid area. If significant clinical improvement does not occur within 15 to 30 minutes, a second dose of 0.25 mg may be administered. If the patient then fails to respond within another 15 to 30 minutes, other therapeutic measures should be considered. The total dose within 4 hours should not exceed 0.5 mg.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Adverse reactions observed with terbutaline sulfate injection are similar to those commonly seen with other sympathomimetic agents. All these reactions are transient in nature and usually do not require treatment. The following table compares adverse reactions seen in patients treated with terbutaline sulfate injection (0.25 mg and 0.5 mg), with those seen in patients treated with epinephrine injection (0.25 mg and 0.5 mg), during eight double-blind crossover studies involving a total of 214 patients.
Incidence (%) of Adverse Reactions | ||||
Terbutaline (%) | Epinephrine (%) | |||
0.25mg N=77 | 0.5 mg N=205 | 0.25mg N=153 | 0.5 mg N=61 | |
Reaction Central Nervous System | ||||
Tremor | 7.8 | 38.0 | 16.3 | 18.0 |
Nervousness | 16.9 | 30.7 | 8.5 | 31.1 |
Dizziness | 1.3 | 10.2 | 7.8 | 3.3 |
Headache | 7.8 | 8.8 | 3.3 | 9.8 |
Drowsiness | 11.7 | 9.8 | 14.4 | 8.2 |
Cardiovascular | ||||
Palpitations | 7.8 | 22.9 | 7.8 | 29.5 |
Tachycardia | 1.3 | 1.5 | 2.6 | 0.0 |
Respiratory | ||||
Dyspnea | 0.0 | 2.0 | 2.0 | 0.0 |
Chest discomfort | 1.3 | 1.5 | 2.6 | 0.0 |
Gastrointestinal | ||||
Nausea/vomiting | 1.3 | 3.9 | 1.3 | 11.5 |
Systemic | ||||
Weakness | 1.3 | 0.5 | 2.6 | 1.6 |
Flushed feeling | 0.0 | 2.4 | 1.3 | 0.0 |
Sweating | 0.0 | 2.4 | 0.0 | 0.0 |
Pain at injection site | 2.6 | 0.5 | 2.6 | 1.6 |
To report SUSPECTED ADVERSE REACTIONS, contact Areva Pharamceuticals Inc. at 1-855-853-4760 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
The concomitant use of terbutaline sulfate injection with other sympathomimetic agents is not recommended, since the combined effect on the cardiovascular system may be deleterious to the patient.
Terbutaline sulfate USP, the active ingredient of terbutaline sulfate injection, is a beta-adrenergic agonist bronchodilator available as a sterile, nonpyrogenic, aqueous solution in vials, for subcutaneous administration. Each milliliter of solution contains 1 mg of terbutaline sulfate USP (0.82 mg of the free base), sodium chloride for isotonicity, and hydrochloric acid for adjustment to a target pH of 4. Terbutaline sulfate is (±)-α-[(tert-butylamino)methyl]-3,5-dihydroxybenzyl alcohol sulfate (2:1) (salt). The molecular formula is (C12H19NO3)2•H2SO4 and the structural formula is:
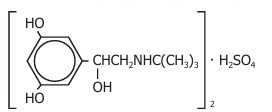
Terbutaline sulfate USP is a white to gray-white crystalline powder. It is odorless or has a faint odor of acetic acid. It is soluble in water and in 0.1N hydrochloric acid, slightly soluble in methanol, and insoluble in chloroform. Its molecular weight is 548.65.