Theophylline
Theophylline Prescribing Information
Theophylline extended-release tablets are indicated for the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
Taking theophylline extended-release tablets immediately after a high-fat content meal may result in a somewhat higher C
maxand delayed T
maxand somewhat greater extent of absorption. However, the differences are usually not great and this product may normally be administered without regard to meals (see
Theophylline extended-release tablets are recommended for chronic or long-term management and prevention of symptoms, and not for use in treating acute symptoms of asthma and reversible bronchospasm.
The steady-state peak serum theophylline concentration is a function of the dose, the dosing interval, and the rate of theophylline absorption and clearance in the individual patient. Because of marked individual differences in the rate of theophylline clearance, the dose required to achieve a peak serum theophylline concentration in the 10-20 mcg/mL range varies fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance (e.g., 400- 1600 mg/day in adults <60 years old and 10-36 mg/kg/day in children 1-9 years old). For a given population there is no single theophylline dose that will provide both safe and effective serum concentrations for all patients. Administration of the median theophylline dose required to achieve a therapeutic serum theophylline concentration in a given population may result in either sub-therapeutic or potentially toxic serum theophylline concentrations in individual patients. For example, at a dose of 900 mg/d in adults <60 years or 22 mg/kg/d in children 1-9 years, the steady-state peak serum theophylline concentration will be <10 mcg/mL in about 30% of patients, 10-20 mcg/mL in about 50% and 20-30 mcg/mL in about 20% of patients.
Transient caffeine-like adverse effects and excessive serum concentrations in slow metabolizers can be avoided in most patients by starting with a sufficiently low dose and slowly increasing the dose,
If the patient's symptoms are well controlled, there are no apparent adverse effects, and no intervening factors that might alter dosage requirements (see
Theophylline distributes poorly into body fat, therefore, mg/kg dose should be calculated on the basis of ideal body weight.
Table V contains theophylline dosing titration schema recommended for patients in various age groups and clinical circumstances. Table VI contains recommendations for theophylline dosage adjustment based upon serum theophylline concentrations.
A.
Titration Step | Children < 45 kg | Children > 45 kg and adults | |
| 1 | Starting Dosage | 12-14 mg/kg/day up to a maximum of 300 mg/day divided Q12 hrs* | 300 mg/day divided Q12 hrs* |
| 2 | After 3 days, if tolerated increase dose to: | 16 mg/kg/day up to a maximum of 400 mg/day divided Q12 hrs* | 400 mg/day divided Q12 hrs* |
| 3 | After 3 more days, if tolerated increase dose to: | 20 mg/kg/day up to a maximum of 600 mg/day divided Q12 hrs* | 600 mg/day divided Q12 hrs* |
B.
In children 6-15 years of age, the final theophylline dose should not exceed 16 mg/kg/day up to a maximum of 400 mg/day in the presence of risk factors for reduced theophylline clearance (see
* Patients with more rapid metabolism, clinically identified by higher than average dose requirements, should receive a smaller dose more frequently (every 8 hours) to prevent breakthrough symptoms resulting from low trough concentrations before the next dose.
Peak Serum Concentration | Dosage Adjustment |
| <9.9 mcg/mL | If symptoms are not controlled and current dosage is tolerated, increase dose about 25%. Recheck serum concentration after three days for further dosage adjustment. |
| 10 to 14.9 mcg/mL | If symptoms are controlled and current dosage is tolerated, maintain dose and recheck serum concentration at 6-12 month intervals.¶ If symptoms are not controlled and current dosage is tolerated consider adding additional medication(s) to treatment regimen. |
| 15-19.9 mcg/mL | Consider 10% decrease in dose to provide greater margin of safety even if current dosage is tolerated.¶ |
| 20-24.9 mcg/mL | Decrease dose by 25% even if no adverse effects are present. Recheck serum concentration after 3 days to guide further dosage adjustment. |
| 25-30 mcg/mL | Skip next dose and decrease subsequent doses at least 25% even if no adverse effects are present. Recheck serum concentration after 3 days to guide further dosage adjustment. If symptomatic, consider whether overdose treatment is indicated (see recommendations for chronic over dosage). |
| >30 mcg/mL | Treat overdose as indicated (see recommendations for chronic overdosage). If theophylline is subsequently resumed, decrease dose by at least 50% and recheck serum concentration after 3 days to guide further dosage adjustment. |
¶ Dose reduction and/or serum theophylline concentration measurement is indicated whenever adverse effects are present, physiologic abnormalities that can reduce theophylline clearance occur (e.g., sustained fever), or a drug that interacts with theophylline is added or discontinued (see
min) obtained following conversion to once-daily dosing may be lower (especially in high clearance patients) and the peak concentration (C
max) may be higher (especially in low clearance patients) than that obtained with q12h dosing. If symptoms recur, or signs of toxicity appear during the once-daily dosing interval, dosing on the q12h basis should be reinstituted.
It is essential that serum theophylline concentrations be monitored before and after transfer to once-daily dosing.
Food and posture, along with changes associated with circadian rhythm, may influence the rate of absorption and / or clearance rates of theophylline from extended-release dosage forms administered at night. The exact relationship of these and other factors to nighttime serum concentrations and the clinical significance of such findings require additional study. Therefore, it is not recommended that theophylline extended-release once-daily dosing be administered at night.
Theophylline extended-release tablets are contraindicated in patients with a history of hypersensitivity to theophylline or other components in the product.
Adverse reactions associated with theophylline are generally mild when peak serum theophylline concentrations are <20 mcg/mL and mainly consist of transient caffeine-like adverse effects such as nausea, vomiting, headache, and insomnia. When peak serum theophylline concentrations exceed 20 mcg/mL, however, theophylline produces a wide range of adverse reactions including persistent vomiting, cardiac arrhythmias, and intractable seizures which can be lethal (see
| Percentage of patients reported with sign or symptoms | ||||
| Acute Overdose (Large Single Ingestion) | Chronic Overdosage (Multiple Excessive Doses) | |||
| Sign / Symptom | Study 1 (n = 157) | Study 2 (n = 14) | Study 1 (n = 92) | Study 2 (n = 102) |
| Asymptomatic | NR** | 0 | NR** | 6 |
| Gastrointestinal | ||||
| Vomiting | 73 | 93 | 30 | 61 |
| Abdominal Pain | NR** | 21 | NR** | 12 |
| Diarrhea | NR** | 0 | NR** | 14 |
| Hematemesis | NR** | 0 | NR** | 2 |
| Metabolic/Other | ||||
| Hypokalemia | 85 | 79 | 44 | 43 |
| Hyperglycemia | 98 | NR** | 18 | NR** |
| Acid/base disturbance | 34 | 21 | 9 | 5 |
| Rhabdomyolysis | NR** | 7 | NR** | 0 |
| Cardiovascular | ||||
| Sinus tachycardia | 100 | 86 | 100 | 62 |
| Other Supraventricular Tachycardias | 2 | 21 | 12 | 14 |
| Ventricular premature beats | 3 | 21 | 10 | 19 |
| Atrial fibrillation or flutter | 1 | NR** | 12 | NR** |
| Multifocal atrial tachycardia | 0 | NR** | 2 | NR** |
| Ventricular arrhythmias hemodynamic instability | 7 | 14 | 40 | 0 |
| Hypotension/shock | NR** | 21 | NR** | 8 |
| Neurologic | ||||
| Nervousness | NR** | 64 | NR** | 21 |
| Tremors | 38 | 29 | 16 | 14 |
| Disorientation | NR** | 7 | NR** | 11 |
| Seizures | 5 | 14 | 14 | 5 |
| Death | 3 | 21 | 10 | 4 |
* These data are derived from two studies in patients with serum theophylline concentrations >30 mcg/mL. In the first study (Study #1 - Shanon, Ann Intern Med 1993; 119:1161-67), data were prospectively collected from 249 consecutive cases of theophylline toxicity referred to a regional poison center for consultation. In the second study (Study #2 - Sessler, Am J Med 1990;88:567-76), data were retrospectively collected from 116 cases with serum theophylline concentrations >30 mcg/mL among 6000 blood samples obtained for measurement of serum theophylline concentrations in three emergency departments. Differences in the incidence of manifestations of theophylline toxicity between the two studies may reflect sample selection as a result of study design (e.g., in Study #1, 48% of the patients had acute intoxications versus only 10% in Study #2) and different methods of reporting results.
** NR = Not reported in a comparable manner.
The drugs listed in Table II have the potential to produce clinically significant pharmacodynamic or pharmacokinetic interactions with theophylline. The information in the "Effect" column of Table II assumes that the interacting drug is being added to a steady-state theophylline regimen. If theophylline is being initiated in a patient who is already taking a drug that inhibits theophylline clearance (e.g., cimetidine, erythromycin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be smaller. Conversely, if theophylline is being initiated in a patient who is already taking a drug that enhances theophylline clearance (e.g., rifampin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be larger. Discontinuation of a concomitant drug that increases theophylline clearance will result in accumulation of theophylline to potentially toxic levels, unless the theophylline dose is appropriately reduced. Discontinuation of a concomitant drug that inhibits theophylline clearance will result in decreased serum theophylline concentrations, unless the theophylline dose is appropriately increased.
The drugs listed in Table III have either been documented not to interact with theophylline or do not produce a clinically significant interaction (i.e., <15% change in theophylline clearance).
The listing of drugs in Tables II and III are current as of February 9, 1995. New interactions are continuously being reported for theophylline, especially with new chemical entities.
| Drug | Type of Interaction | Effect** |
| Adenosine | Theophylline blocks adenosine receptors. | Higher doses of adenosine may be required to achieve desired effect. |
| Alcohol | A single large dose of alcohol (3 mL/kg of whiskey) decreases theophylline clearance for up to 24 hours. | 30% increase |
| Allopurinol | Decreases Theophylline clearance at allopurinol doses 600 mg/day. | 25% increase |
| Aminoglutethimide | Increases theophylline clearance by induction of microsomal enzyme activity. | 25% decrease |
| Carbamazepine | Similar to aminoglutethimide. | 30% decrease |
| Cimetidine | Decreases theophylline clearance by inhibiting cytochrome P450 1A2. | 70% increase |
| Ciprofloxacin | Similar to cimetidine. | 40% increase |
| Clarithromycin | Similar to erythromycin. | 25% increase |
| Diazepam | Benzodiazepines increase CNS concentrations of adenosine, a potent CNS depressant, while theophylline blocks adenosine receptors. | Larger diazepam doses may be required to produce desired level of sedation. Discontinuation of Theophylline without reduction of diazepam dose may result in respiratory depression. |
| Disulfiram | Decreases theophylline clearance by inhibiting hydroxylation and demethylation. | 50% increase |
| Enoxacin | Similar to cimetidine. | 300% increase |
| Ephedrine | Synergistic CNS effects. | Increased frequency of nausea, nervousness, and insomnia. |
| Erythromycin | Erythromycin metabolite decreases theophylline clearance by inhibiting cytochrome P450 3A3. | 35% increase. Erythromycin steady- state serum concentrations decrease by a similar amount. |
| Estrogen | Estrogen containing oral contraceptives decrease theophylline clearance in a dose- dependent fashion. The effect of progesterone on theophylline clearance is unknown. | 30% increase |
| Flurazepam | Similar to diazepam. | Similar to diazepam. |
| Fluvoxamine | Similar to cimetidine. | Similar to cimetidine. |
| Halothane | Halothane sensitizes the myocardium to catecholamines, theophylline increases release of endogenous catecholamines. | Increased risk of ventricular arrhythmias. |
| Interferon, human recombinant alpha-A | Decreases theophylline clearance. | 100% increase |
| Isoproterenol (IV) | Increases theophylline clearance. | 20% decrease |
| Ketamine | Pharmacologic. | May lower theophylline seizure threshold |
| Lithium | Theophylline increases renal lithium clearance. | Lithium dose required to achieve a therapeutic serum concentration increased an average of 60%. |
| Lorazepam | Similar to diazepam. | Similar to diazepam. |
| Methotrexate (MTX) | Decreases theophylline clearance. | 20% increase after low dose MTX, higher dose MTX may have a greater effect. |
| Mexiletine | Similar to disulfiram. | 80% increase |
| Midazolam | Similar to diazepam. | Similar to diazepam. |
| Moricizine | Increases theophylline clearance. | 25% decrease |
| Pancuronium | Theophylline may antagonize non-depolarizing neuromuscular blocking effects; possibly due to phosphodiesterase inhibition. | Larger dose of pancuronium may be required to achieve neuromuscular blockade. |
| Pentoxifylline | Decreases theophylline clearance. | 30% increase |
| Phenobarbital (PB) | Similar to aminoglutethimide. | 25% decrease after two weeks of concurrent PB. |
| Phenytoin | Phenytoin increases theophylline clearance by increasing microsomal enzyme activity. Theophylline decreases phenytoin absorption. | Serum theophylline and phenytoin concentrations decrease about 40%. |
| Propafenone | Decreases theophylline clearance and pharmacologic interaction. | 40% increase. Beta-2 blocking effect may decrease efficacy of theophylline. |
| Propranolol | Similar to cimetidine and pharmacologic interaction. | 100% increase. Beta-2 blocking effect may decrease efficacy of theophylline. |
| Rifampin | Increases theophylline clearance by increasing cytochrome P450 1A2 and 3A3 activity. | 20-40% decrease |
| St. JohnsWort (Hypericum Perforatum) | Decrease in theophylline plasma concentrations. | Higher doses of theophylline may be required to achieve desired effect. Stopping St. Johns Wort may result in theophylline toxicity. |
| Sulfinpyrazone | Increase theophylline clearance by increasing demethylation and hydroxylation. Decreases renal clearance of theophylline. | 20% decrease |
Tacrine | Similar to cimetidine, also increases renal clearance of theophylline. | 90% increase |
| Thiabendazole | Decreases theophylline clearance. | 190% increase |
| Ticlopidine | Decreases theophylline clearance. | 60% increase |
| Troleandomycin | Similar to erythromycin. | 33-100% increase depending on troleandomycin dose. |
| Verapamil | Similar to disulfiram. | 20% increase |
| albuterol, systemic and inhaled | mebendazole |
| amoxicillin | medroxyprogesterone |
| ampicillin, with or without | methylprednisolone |
| sulbactam | metronidazole |
| atenolol | metoprolol |
| azithromycin | nadolol |
| caffeine, dietary ingestion | nifedipine |
| cefaclor | nizatidine |
co-trimoxazole (trimethoprim and sulfamethoxazole) | norfloxacin ofloxacin |
| diltiazem | omeprazole |
| dirithromycin | prednisone, prednisolone |
| enflurane | ranitidine |
| famotidine | rifabutin |
| felodipine | roxithromycin |
| finasteride | Sorbitol (purgative doses do not inhibit |
| hydrocortisone | theophylline absorption) |
| isoflurane | sucralfate |
| isoniazid | terbutaline, systemic |
| isradipine | terfenadine |
| influenza vaccine | tetracycline |
| ketoconazole | tocainide |
| lomefloxacin |
maxand delayed T
maxand a somewhat greater extent of absorption when compared to taking it in the fasting state. The influence of the type and amount of other foods, as well as the time interval between drug and food, has not been studied.
Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1
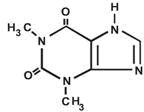
C
7H
8N
4O
2 M.W. 180.17.
This product allows a 12-hour dosing interval for a majority of patients and a 24-hour dosing interval for selected patients (see
Each extended-release tablet for oral administration contains either 300 mg or 450 mg of anhydrous theophylline. Tablets also contain as inactive ingredients: hypromellose, lactose monohydrate, magnesium stearate, povidone and colloidal silicone dioxide.