Ticagrelor Prescribing Information
- Ticagrelor, like other antiplatelet agents, can cause significant, sometimes fatal bleeding (
5.1 Risk of BleedingDrugs that inhibit platelet function including ticagrelor increase the risk of bleeding
[see Warnings and Precautions (5.2)andAdverse Reactions (6.1)].Patients treated for acute ischemic stroke or TIAPatients at NIHSS > 5 and patients receiving thrombolysis were excluded from THALES and use of ticagrelor in such patients is not recommended.
,5.1 Risk of BleedingDrugs that inhibit platelet function including ticagrelor increase the risk of bleeding
[see Warnings and Precautions (5.2)andAdverse Reactions (6.1)].Patients treated for acute ischemic stroke or TIAPatients at NIHSS > 5 and patients receiving thrombolysis were excluded from THALES and use of ticagrelor in such patients is not recommended.
).6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ticagrelor has been evaluated for safety in more than 58,000 patients.
Bleeding in PLATO (Reduction in risk of thrombotic events in ACS)Figure 1 is a plot of time to the first non-CABG major bleeding event.
Figure 1: Kaplan-Meier estimate of time to first non-CABG PLATO-defined major bleeding event (PLATO)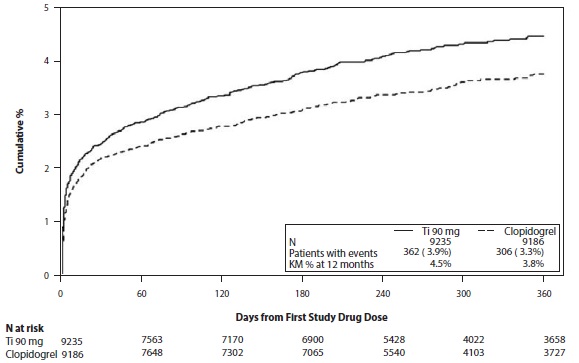
Frequency of bleeding in PLATO is summarized in Tables 1 and 2. About half of the non-CABG major bleeding events were in the first 30 days.
Table 1: Non-CABG related bleeds (PLATO)Ticagrelor*N=9,235ClopidogrelN=9,186n (%) patients with eventn (%) patients with eventPLATO Major + Minor
713 (7.7)
567 (6.2)
Major
362 (3.9)
306 (3.3)
Fatal/Life-threatening
171 (1.9)
151 (1.6)
Fatal
15 (0.2)
16 (0.2)
Intracranial hemorrhage (Fatal/Life-threatening)
26 (0.3)
15 (0.2)
PLATO Minor bleed:requires medical intervention to stop or treat bleeding.PLATO Major bleed:any one of the following: fatal; intracranial; intrapericardial with cardiac tamponade; hypovolemic shock or severe hypotension requiring intervention; significantly disabling (e.g., intraocular with permanent vision loss); associated with a decrease in Hb of at least 3 g/dL (or a fall in hematocrit (Hct) of at least 9%); transfusion of 2 or more units.PLATO Major bleed, fatal/life-threatening:any major bleed as described above and associated with a decrease in Hb of more than 5 g/dL (or a fall in hematocrit (Hct) of at least 15%); transfusion of 4 or more units.Fatal:A bleeding event that directly led to death within 7 days.*90 mg BID
No baseline demographic factor altered the relative risk of bleeding with ticagrelor compared to clopidogrel.
In PLATO, 1,584 patients underwent CABG surgery. The percentages of those patients who bled are shown in Figure 2 and Table 2.
Figure 2: ‘Major fatal/life-threatening’ CABG-related bleeding by days from last dose of study drug toCABG procedure (PLATO)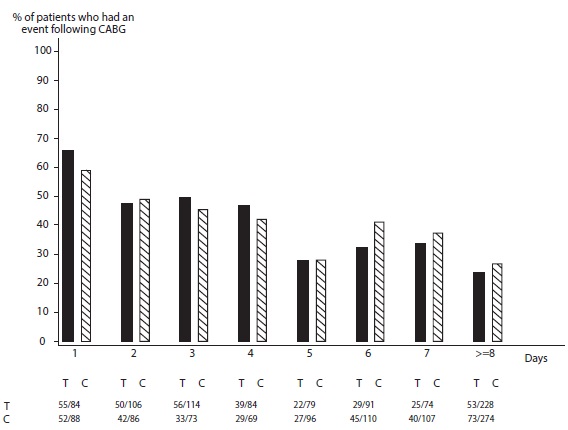
X-axis is days from last dose of study drug prior to CABG.
The PLATO protocol recommended a procedure for withholding study drug prior to CABG or other major surgery without unblinding. If surgery was elective or non-urgent, study drug was interrupted temporarily, as follows: If local practice was to allow antiplatelet effects to dissipate before surgery, capsules (blinded clopidogrel) were withheld 5 days before surgery and tablets (blinded ticagrelor) were withheld for a minimum of 24 hours and a maximum of 72 hours before surgery. If local practice was to perform surgery without waiting for dissipation of antiplatelet effects capsules and tablets were withheld 24 hours prior to surgery and use of aprotinin or other hemostatic agents was allowed. If local practice was to use IPA monitoring to determine when surgery could be performed both the capsules and tablets were withheld at the same time and the usual monitoring procedures followed.
T = Ticagrelor; C = Clopidogrel.
Table 2: CABG-related bleeding (PLATO)Ticagrelor*N=770ClopidogrelN=814n (%) patients with eventn (%) patients with eventPLATO Total Major
626 (81.3)
666 (81.8)
Fatal/Life-threatening
337 (43.8)
350 (43.0)
Fatal
6 (0.8)
7 (0.9)
PLATO Major bleed:any one of the following: fatal; intracranial; intrapericardial with cardiac tamponade; hypovolemic shock or severe hypotension requiring intervention; significantly disabling (e.g., intraocular with permanent vision loss); associated with a decrease in Hb of at least 3 g/dL (or a fall in hematocrit (Hct) of at least 9%); transfusion of 2 or more units.PLATO Major bleed, fatal/life-threatening:any major bleed as described above and associated with a decrease in Hb of more than 5 g/dL (or a fall in hematocrit (Hct) of at least 15%); transfusion of 4 or more units.*90 mg BID
When antiplatelet therapy was stopped 5 days before CABG, major bleeding occurred in 75% of ticagrelor treated patients and 79% on clopidogrel.
Other Adverse Reactions in PLATOAdverse reactions that occurred at a rate of 4% or more in PLATO are shown in Table 3.
Table 3: Percentage of patients reporting non-hemorrhagic adverse reactions at least 4% or more in either group and more frequently on ticagrelor (PLATO)Ticagrelor*N=9,235ClopidogrelN=9,186Dyspnea
13.8
7.8
Dizziness
4.5
3.9
Nausea
4.3
3.8
*90 mg BID
Bleeding in PEGASUS (Secondary Prevention in Patients with a History of Myocardial Infarction)Overall outcome of bleeding events in the PEGASUS study are shown in Table 4.
Table 4: Bleeding events (PEGASUS)Ticagrelor*
N=6,958Placebo
N=6,996Events/1,000 patient yearsEvents/1,000 patient yearsTIMI Major
8
3Fatal
1 1 Intracranial hemorrhage
2
1TIMI Major or Minor
11 5 TIMI Major:Fatal bleeding, OR any intracranial bleeding, OR clinically overt signs of hemorrhage associated with a drop in hemoglobin (Hgb) of ≥ 5 g/dL, or a fall in hematocrit (Hct) of ≥ 15%.Fatal:A bleeding event that directly led to death within 7 days.TIMI Minor:Clinically apparent with 3 g/dL to 5 g/dL decrease in hemoglobin.*60 mg BID
The bleeding profile of ticagrelor 60 mg compared to aspirin alone was consistent across multiple pre-defined subgroups (e.g., by age, gender, weight, race, geographic region, concurrent conditions, concomitant therapy, stent, and medical history) for TIMI Major and TIMI Major or Minor bleeding events.
Other Adverse Reactions in PEGASUSAdverse reactions that occurred in PEGASUS at rates of 3% or more are shown in Table 5.
Table 5: Non-hemorrhagic adverse reactions reported in > 3.0% of patients in the ticagrelor 60 mg treatment group (PEGASUS)Ticagrelor*
N=6,958Placebo
N=6,996Dyspnea
14.2%
5.5%
Dizziness
4.5%
4.1%
Diarrhea
3.3%
2.5%
*60 mg BID
Bleeding in THEMIS (Prevention of major CV events in patients with CAD and Type 2 Diabetes Mellitus)The Kaplan-Meier curve of time to first TIMI Major bleeding event is presented in Figure 3.
Figure 3: Time to first TIMI Major bleeding event (THEMIS)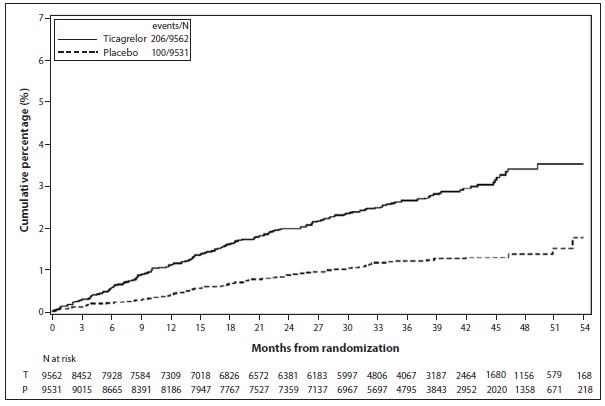
T = Ticagrelor; P = Placebo; N = Number of patients
The bleeding events in THEMIS are shown below in Table 6.
Table 6: Bleeding events (THEMIS)TicagrelorN=9,562PlaceboN=9,531Events/1,000 patient yearsEvents/1,000 patient yearsTIMI Major
9
4
TIMI Major or Minor
12
5
TIMI Major or Minor or Requiring medical attention
46
18
Fatal bleeding
1
0
Intracranial hemorrhage
3
2
Bleeding in THALES (Reduction in risk of stroke in patients with acute ischemic stroke or TIA)The Kaplan-Meier curve of time course of GUSTO severe bleeding events is presented in Figure 4.
Figure 4: Time course of GUSTO severe bleeding events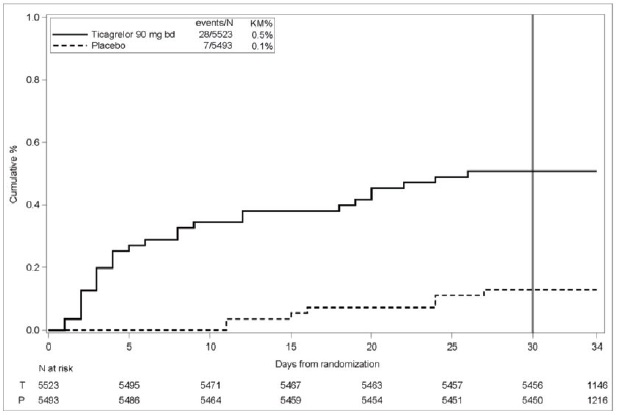
KM%: Kaplan-Meier percentage evaluated at Day 30; T = Ticagrelor; P = placebo; N = Number of patients
GUSTO Severe:Any one of the following: fatal bleeding, intracranial bleeding (excluding asymptomatic hemorrhagic transformations of ischemic brain infarctions and excluding microhemorrhages < 10 mm evident only on gradient-echo magnetic resonance imaging), bleeding that caused hemodynamic compromise requiring intervention (e.g., systolic blood pressure < 90 mmg Hg that required blood or fluid replacement, or vasopressor/inotropic support, or surgical intervention).Intracranial bleeding and fatal bleeding in THALES:In total, there were 21 intracranial hemorrhages (ICHs) for ticagrelor and 6 ICHs for placebo. Fatal bleedings, almost all ICH, occurred in 11 for ticagrelor and in 2 for placebo.BradycardiaIn a Holter substudy of about 3,000 patients in PLATO, more patients had ventricular pauses with ticagrelor (6.0%) than with clopidogrel (3.5%) in the acute phase; rates were 2.2% and 1.6%, respectively, after 1 month. PLATO, PEGASUS, THEMIS and THALES excluded patients at increased risk of bradycardic events (e.g., patients who have sick sinus syndrome, 2ndor 3rddegree AV block, or bradycardic-related syncope and not protected with a pacemaker).
Lab abnormalitiesSerum Uric Acid:
In PLATO, serum uric acid levels increased approximately 0.6 mg/dL from baseline on ticagrelor 90 mg and approximately 0.2 mg/dL on clopidogrel. The difference disappeared within 30 days of discontinuing treatment. Reports of gout did not differ between treatment groups in PLATO (0.6% in each group).
In PEGASUS, serum uric acid levels increased approximately 0.2 mg/dL from baseline on ticagrelor 60 mg and no elevation was observed on aspirin alone. Gout occurred more commonly in patients on ticagrelor than in patients on aspirin alone (1.5%, 1.1%). Mean serum uric acid concentrations decreased after treatment was stopped.
Serum Creatinine:
In PLATO, a > 50% increase in serum creatinine levels was observed in 7.4% of patients receiving ticagrelor 90 mg compared to 5.9% of patients receiving clopidogrel. The increases typically did not progress with ongoing treatment and often decreased with continued therapy. Evidence of reversibility upon discontinuation was observed even in those with the greatest on treatment increases. Treatment groups in PLATO did not differ for renal-related serious adverse events such as acute renal failure, chronic renal failure, toxic nephropathy, or oliguria.
In PEGASUS, serum creatinine concentration increased by > 50% in approximately 4% of patients receiving ticagrelor 60 mg, similar to aspirin alone. The frequency of renal related adverse events was similar for ticagrelor and aspirin alone regardless of age and baseline renal function.

1 
2 
3 
4 - Do not use ticagrelor in patients with active pathological bleeding or a history of intracranial hemorrhage (,
4.1 History of Intracranial HemorrhageTicagrelor tablets are contraindicated in patients with a history of intracranial hemorrhage (ICH) because of a high risk of recurrent ICH in this population
[seeClinical Studies (14.1), (14.2)].).4.2 Active BleedingTicagrelor tablets are contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage
[see Warnings and Precautions (5.1)andAdverse Reactions (6.1)]. - Do not start ticagrelor in patients undergoing urgent coronary artery bypass graft surgery (CABG) (,
5.1 Risk of BleedingDrugs that inhibit platelet function including ticagrelor increase the risk of bleeding
[see Warnings and Precautions (5.2)andAdverse Reactions (6.1)].Patients treated for acute ischemic stroke or TIAPatients at NIHSS > 5 and patients receiving thrombolysis were excluded from THALES and use of ticagrelor in such patients is not recommended.
).6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ticagrelor has been evaluated for safety in more than 58,000 patients.
Bleeding in PLATO (Reduction in risk of thrombotic events in ACS)Figure 1 is a plot of time to the first non-CABG major bleeding event.
Figure 1: Kaplan-Meier estimate of time to first non-CABG PLATO-defined major bleeding event (PLATO)
Frequency of bleeding in PLATO is summarized in Tables 1 and 2. About half of the non-CABG major bleeding events were in the first 30 days.
Table 1: Non-CABG related bleeds (PLATO)Ticagrelor*N=9,235ClopidogrelN=9,186n (%) patients with eventn (%) patients with eventPLATO Major + Minor
713 (7.7)
567 (6.2)
Major
362 (3.9)
306 (3.3)
Fatal/Life-threatening
171 (1.9)
151 (1.6)
Fatal
15 (0.2)
16 (0.2)
Intracranial hemorrhage (Fatal/Life-threatening)
26 (0.3)
15 (0.2)
PLATO Minor bleed:requires medical intervention to stop or treat bleeding.PLATO Major bleed:any one of the following: fatal; intracranial; intrapericardial with cardiac tamponade; hypovolemic shock or severe hypotension requiring intervention; significantly disabling (e.g., intraocular with permanent vision loss); associated with a decrease in Hb of at least 3 g/dL (or a fall in hematocrit (Hct) of at least 9%); transfusion of 2 or more units.PLATO Major bleed, fatal/life-threatening:any major bleed as described above and associated with a decrease in Hb of more than 5 g/dL (or a fall in hematocrit (Hct) of at least 15%); transfusion of 4 or more units.Fatal:A bleeding event that directly led to death within 7 days.*90 mg BID
No baseline demographic factor altered the relative risk of bleeding with ticagrelor compared to clopidogrel.
In PLATO, 1,584 patients underwent CABG surgery. The percentages of those patients who bled are shown in Figure 2 and Table 2.
Figure 2: ‘Major fatal/life-threatening’ CABG-related bleeding by days from last dose of study drug toCABG procedure (PLATO)
X-axis is days from last dose of study drug prior to CABG.
The PLATO protocol recommended a procedure for withholding study drug prior to CABG or other major surgery without unblinding. If surgery was elective or non-urgent, study drug was interrupted temporarily, as follows: If local practice was to allow antiplatelet effects to dissipate before surgery, capsules (blinded clopidogrel) were withheld 5 days before surgery and tablets (blinded ticagrelor) were withheld for a minimum of 24 hours and a maximum of 72 hours before surgery. If local practice was to perform surgery without waiting for dissipation of antiplatelet effects capsules and tablets were withheld 24 hours prior to surgery and use of aprotinin or other hemostatic agents was allowed. If local practice was to use IPA monitoring to determine when surgery could be performed both the capsules and tablets were withheld at the same time and the usual monitoring procedures followed.
T = Ticagrelor; C = Clopidogrel.
Table 2: CABG-related bleeding (PLATO)Ticagrelor*N=770ClopidogrelN=814n (%) patients with eventn (%) patients with eventPLATO Total Major
626 (81.3)
666 (81.8)
Fatal/Life-threatening
337 (43.8)
350 (43.0)
Fatal
6 (0.8)
7 (0.9)
PLATO Major bleed:any one of the following: fatal; intracranial; intrapericardial with cardiac tamponade; hypovolemic shock or severe hypotension requiring intervention; significantly disabling (e.g., intraocular with permanent vision loss); associated with a decrease in Hb of at least 3 g/dL (or a fall in hematocrit (Hct) of at least 9%); transfusion of 2 or more units.PLATO Major bleed, fatal/life-threatening:any major bleed as described above and associated with a decrease in Hb of more than 5 g/dL (or a fall in hematocrit (Hct) of at least 15%); transfusion of 4 or more units.*90 mg BID
When antiplatelet therapy was stopped 5 days before CABG, major bleeding occurred in 75% of ticagrelor treated patients and 79% on clopidogrel.
Other Adverse Reactions in PLATOAdverse reactions that occurred at a rate of 4% or more in PLATO are shown in Table 3.
Table 3: Percentage of patients reporting non-hemorrhagic adverse reactions at least 4% or more in either group and more frequently on ticagrelor (PLATO)Ticagrelor*N=9,235ClopidogrelN=9,186Dyspnea
13.8
7.8
Dizziness
4.5
3.9
Nausea
4.3
3.8
*90 mg BID
Bleeding in PEGASUS (Secondary Prevention in Patients with a History of Myocardial Infarction)Overall outcome of bleeding events in the PEGASUS study are shown in Table 4.
Table 4: Bleeding events (PEGASUS)Ticagrelor*
N=6,958Placebo
N=6,996Events/1,000 patient yearsEvents/1,000 patient yearsTIMI Major
8
3Fatal
1 1 Intracranial hemorrhage
2
1TIMI Major or Minor
11 5 TIMI Major:Fatal bleeding, OR any intracranial bleeding, OR clinically overt signs of hemorrhage associated with a drop in hemoglobin (Hgb) of ≥ 5 g/dL, or a fall in hematocrit (Hct) of ≥ 15%.Fatal:A bleeding event that directly led to death within 7 days.TIMI Minor:Clinically apparent with 3 g/dL to 5 g/dL decrease in hemoglobin.*60 mg BID
The bleeding profile of ticagrelor 60 mg compared to aspirin alone was consistent across multiple pre-defined subgroups (e.g., by age, gender, weight, race, geographic region, concurrent conditions, concomitant therapy, stent, and medical history) for TIMI Major and TIMI Major or Minor bleeding events.
Other Adverse Reactions in PEGASUSAdverse reactions that occurred in PEGASUS at rates of 3% or more are shown in Table 5.
Table 5: Non-hemorrhagic adverse reactions reported in > 3.0% of patients in the ticagrelor 60 mg treatment group (PEGASUS)Ticagrelor*
N=6,958Placebo
N=6,996Dyspnea
14.2%
5.5%
Dizziness
4.5%
4.1%
Diarrhea
3.3%
2.5%
*60 mg BID
Bleeding in THEMIS (Prevention of major CV events in patients with CAD and Type 2 Diabetes Mellitus)The Kaplan-Meier curve of time to first TIMI Major bleeding event is presented in Figure 3.
Figure 3: Time to first TIMI Major bleeding event (THEMIS)
T = Ticagrelor; P = Placebo; N = Number of patients
The bleeding events in THEMIS are shown below in Table 6.
Table 6: Bleeding events (THEMIS)TicagrelorN=9,562PlaceboN=9,531Events/1,000 patient yearsEvents/1,000 patient yearsTIMI Major
9
4
TIMI Major or Minor
12
5
TIMI Major or Minor or Requiring medical attention
46
18
Fatal bleeding
1
0
Intracranial hemorrhage
3
2
Bleeding in THALES (Reduction in risk of stroke in patients with acute ischemic stroke or TIA)The Kaplan-Meier curve of time course of GUSTO severe bleeding events is presented in Figure 4.
Figure 4: Time course of GUSTO severe bleeding events
KM%: Kaplan-Meier percentage evaluated at Day 30; T = Ticagrelor; P = placebo; N = Number of patients
GUSTO Severe:Any one of the following: fatal bleeding, intracranial bleeding (excluding asymptomatic hemorrhagic transformations of ischemic brain infarctions and excluding microhemorrhages < 10 mm evident only on gradient-echo magnetic resonance imaging), bleeding that caused hemodynamic compromise requiring intervention (e.g., systolic blood pressure < 90 mmg Hg that required blood or fluid replacement, or vasopressor/inotropic support, or surgical intervention).Intracranial bleeding and fatal bleeding in THALES:In total, there were 21 intracranial hemorrhages (ICHs) for ticagrelor and 6 ICHs for placebo. Fatal bleedings, almost all ICH, occurred in 11 for ticagrelor and in 2 for placebo.BradycardiaIn a Holter substudy of about 3,000 patients in PLATO, more patients had ventricular pauses with ticagrelor (6.0%) than with clopidogrel (3.5%) in the acute phase; rates were 2.2% and 1.6%, respectively, after 1 month. PLATO, PEGASUS, THEMIS and THALES excluded patients at increased risk of bradycardic events (e.g., patients who have sick sinus syndrome, 2ndor 3rddegree AV block, or bradycardic-related syncope and not protected with a pacemaker).
Lab abnormalitiesSerum Uric Acid:
In PLATO, serum uric acid levels increased approximately 0.6 mg/dL from baseline on ticagrelor 90 mg and approximately 0.2 mg/dL on clopidogrel. The difference disappeared within 30 days of discontinuing treatment. Reports of gout did not differ between treatment groups in PLATO (0.6% in each group).
In PEGASUS, serum uric acid levels increased approximately 0.2 mg/dL from baseline on ticagrelor 60 mg and no elevation was observed on aspirin alone. Gout occurred more commonly in patients on ticagrelor than in patients on aspirin alone (1.5%, 1.1%). Mean serum uric acid concentrations decreased after treatment was stopped.
Serum Creatinine:
In PLATO, a > 50% increase in serum creatinine levels was observed in 7.4% of patients receiving ticagrelor 90 mg compared to 5.9% of patients receiving clopidogrel. The increases typically did not progress with ongoing treatment and often decreased with continued therapy. Evidence of reversibility upon discontinuation was observed even in those with the greatest on treatment increases. Treatment groups in PLATO did not differ for renal-related serious adverse events such as acute renal failure, chronic renal failure, toxic nephropathy, or oliguria.
In PEGASUS, serum creatinine concentration increased by > 50% in approximately 4% of patients receiving ticagrelor 60 mg, similar to aspirin alone. The frequency of renal related adverse events was similar for ticagrelor and aspirin alone regardless of age and baseline renal function.

1 
2 
3 
4 - If possible, manage bleeding without discontinuing ticagrelor. Stopping ticagrelor increases the risk of subsequent cardiovascular events ().
5.2 Discontinuation of Ticagrelor in Patients Treated for Coronary Artery DiseaseDiscontinuation of ticagrelor will increase the risk of myocardial infarction, stroke, and death in patients being treated for coronary artery disease. If ticagrelor must be temporarily discontinued (e.g., to treat bleeding or for significant surgery), restart it as soon as possible. When possible, interrupt therapy with ticagrelor for five days prior to surgery that has a major risk of bleeding. Resume ticagrelor as soon as hemostasis is achieved.
Ticagrelor Tablets, 90 mg are supplied as yellow colored, round, biconvex film-coated tablets marked with “A” above “11” on one side and plain on the other side.
The following adverse reactions are also discussed elsewhere in the labeling:
- Bleeding [see]
5.1 Risk of BleedingDrugs that inhibit platelet function including ticagrelor increase the risk of bleeding
[see Warnings and Precautions (5.2)andAdverse Reactions (6.1)].Patients treated for acute ischemic stroke or TIAPatients at NIHSS > 5 and patients receiving thrombolysis were excluded from THALES and use of ticagrelor in such patients is not recommended.
- Dyspnea [see]
5.3 DyspneaIn clinical trials, about 14% (PLATO and PEGASUS) to 21% (THEMIS) of patients treated with ticagrelor developed dyspnea. Dyspnea was usually mild to moderate in intensity and often resolved during continued treatment but led to study drug discontinuation in 0.9% (PLATO), 1.0% (THALES), 4.3% (PEGASUS), and 6.9% (THEMIS) of patients.
In a substudy of PLATO, 199 subjects underwent pulmonary function testing irrespective of whether they reported dyspnea. There was no indication of an adverse effect on pulmonary function assessed after one month or after at least 6 months of chronic treatment.
If a patient develops new, prolonged, or worsened dyspnea that is determined to be related to ticagrelor, no specific treatment is required; continue ticagrelor without interruption if possible. In the case of intolerable dyspnea requiring discontinuation of ticagrelor, consider prescribing another antiplatelet agent.
Ticagrelor tablets contain ticagrelor, a cyclopentyltriazolopyrimidine, inhibitor of platelet activation and aggregation mediated by the P2Y12 ADP-receptor. Chemically it is (1
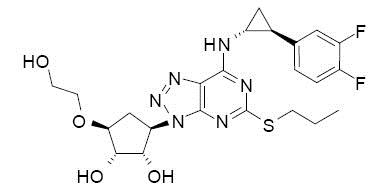
Ticagrelor is a crystalline powder with an aqueous solubility of approximately 10 mcg/mL at room temperature.
Ticagrelor 90 mg tablets for oral administration contain 90 mg of ticagrelor and the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, iron oxide yellow, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, sodium starch glycolate, talc and titanium dioxide.
Ticagrelor Tablets,
They are available as follows:
Bottles of 60: NDC 69238-1134-6
Bottles of 100: NDC 69238-1134-1
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [see USP controlled room temperature].
Ticagrelor and its major metabolite reversibly interact with the platelet P2Y12 ADP-receptor to prevent signal transduction and platelet activation. Ticagrelor and its active metabolite are approximately equipotent.