Timolol Maleate
Timolol Maleate Prescribing Information
Timolol Maleate ophthalmic solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Timolol Maleate ophthalmic solution is available in concentrations of 0.25% and 0.5%. The usual starting dose is one drop of the 0.25% Timolol solution in the affected eye(s) twice a day. If the clinical response is not adequate, the dosage may be changed to one drop of 0.5% solution in the affected eye(s) twice a day.
Since in some patients the pressure-lowering response to timolol may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with timolol.
If the intraocular pressure is maintained at satisfactory levels, the dosage schedule may be changed to one drop once a day in the affected eye(s). Because of diurnal variations in intraocular pressure, satisfactory response to the once-a-day dose is best determined by measuring the intraocular pressure at different times during the day.
Dosages above one drop of a 0.5% Timolol solution twice a day generally have not been shown to produce further reduction in intraocular pressure. If the patient’s intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy with other agent(s) for lowering intraocular pressure can be instituted. The concomitant use of two topical beta-adrenergic blocking agents is not recommended (see
Although timolol used alone has little or no effect on pupil size, mydriasis resulting from concomitant therapy with timolol and epinephrine has been reported occasionally.
Timolol Maleate is contraindicated in patients with (1) bronchial asthma; (2) a history of bronchial asthma; (3) severe chronic obstructive pulmonary disease (see
As with many topically applied ophthalmic drugs, this drug is absorbed systemically.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of adrenergic agonists.
As with many topically applied ophthalmic drugs, this drug is absorbed systemically.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of adrenergic agonists.
The most frequently reported adverse experiences have been burning and stinging upon instillation (approximately one in eight patients).
The following additional adverse experiences have been reported less frequently with ocular administration of this or other Timolol Maleate formulations:
BODY AS A WHOLE: Headache, asthenia/fatigue, and chest pain.
CARDIOVASCULAR: Bradycardia, arrhythmia, hypotension, hypertension, syncope, heart block, cerebral vascular accident, cerebral ischemia, cardiac failure, worsening of angina pectoris, palpitation, cardiac arrest, pulmonary edema, edema, claudication, Raynaud’s phenomenon, and cold hands and feet.
DIGESTIVE: Nausea, diarrhea, dyspepsia, anorexia, and dry mouth.
IMMUNOLOGIC: Systemic lupus erythematosus.
NERVOUS SYSTEM/PSYCHIATRIC: Dizziness, increase in signs and symptoms of myasthenia gravis, paresthesia, somnolence, insomnia, nightmares, behavioral changes and psychic disturbances including depression, confusion, hallucinations, anxiety, disorientation, nervousness, and memory loss.
SKIN: Alopecia and psoriasiform rash or exacerbation of psoriasis.
HYPERSENSITIVITY: Signs and symptoms of systemic allergic reactions, including anaphylaxis, angioedema, urticaria, and localized and generalized rash.
RESPIRATORY: Bronchospasm (predominantly in patients with pre-existing bronchospastic disease), respiratory failure, dyspnea, nasal congestion, cough and upper respiratory infections.
ENDOCRINE: Masked symptoms of hypoglycemia in diabetic patients (see
As with many topically applied ophthalmic drugs, this drug is absorbed systemically.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of adrenergic agonists.
SPECIAL SENSES: Signs and symptoms of ocular irritation including conjunctivitis, blepharitis, keratitis, ocular pain, discharge (e.g., crusting), foreign body sensation, itching and tearing, and dry eyes; ptosis; decreased corneal sensitivity; cystoid macular edema; visual disturbances including refractive changes and diplopia; pseudopemphigoid; choroidal detachment following filtration surgery (see
Because of potential effects of beta-adrenergic blocking agents on blood pressure and pulse, these agents should be used with caution in patients with cerebrovascular insufficiency. If signs or symptoms suggesting reduced cerebral blood flow develop following initiation of therapy with timolol, alternative therapy should be considered.
There have been reports of bacterial keratitis associated with the use of multiple dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PRECAUTIONS, Information for Patients).
Choroidal detachment after filtration procedures has been reported with the administration of aqueous suppressant therapy (e.g., timolol).
UROGENITAL: Retroperitoneal fibrosis, decreased libido, impotence, and Peyronie’s disease.
The following additional adverse effects have been reported in clinical experience with ORAL Timolol Maleate or other ORAL beta-blocking agents and may be considered potential effects of ophthalmic Timolol Maleate:
Although timolol used alone has little or no effect on pupil size, mydriasis resulting from concomitant therapy with timolol and epinephrine has been reported occasionally.
Because of potential effects of beta-adrenergic blocking agents on blood pressure and pulse, these agents should be used with caution in patients with cerebrovascular insufficiency. If signs or symptoms suggesting reduced cerebral blood flow develop following initiation of therapy with timolol, alternative therapy should be considered.
There have been reports of bacterial keratitis associated with the use of multiple dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PRECAUTIONS, Information for Patients).
Choroidal detachment after filtration procedures has been reported with the administration of aqueous suppressant therapy (e.g., timolol).
Timolol Maleate ophthalmic solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical
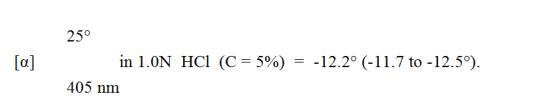
Its molecular formula is C13 H24 N4 O3 S • C4 H4 O4 and its structural formula is:
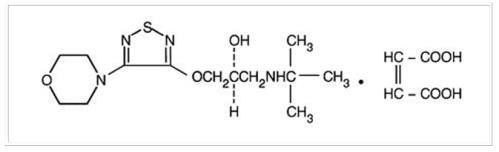
Timolol Maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol Maleate ophthalmic solution is stable at room temperature.
Timolol Maleate ophthalmic solution is supplied as a sterile, isotonic, buffered, aqueous solution of Timolol Maleate in two dosage strengths: Each mL, for ophthalmic administration, of 0.25% solution contains 2.5 mg of timolol (3.4 mg of Timolol Maleate). The pH of the solution is approximately 7.0. Each mL, for ophthalmic administration, of 0.5% solution contains 5 mg of timolol (6.8 mg of Timolol Maleate). Inactive ingredients: monobasic and dibasic sodium phosphate; hydrochloric acid and/or sodium hydroxide to adjust pH; and purified water. Benzalkonium chloride 0.01% is added as preservative.