Tizanidine
Tizanidine Prescribing Information
- Indications and Usage () 11/2024
1 INDICATIONS AND USAGETizanidine is a central alpha-2-adrenergic agonist indicated for the treatment of spasticity.
Tizanidine is indicated for the treatment of spasticity in adults.
- Dosage and Administration (,2.1 Recommended Evaluation and Testing Before and After Initiating TizanidineMonitoring of aminotransferase levels is recommended at baseline and 1 month after maximum dose is achieved[see Warnings and Precautions ].,2.2 Recommended DosageThe recommended starting dose is 2 mg by mouth every 6 to 8 hours, as needed, to a maximum of three doses in 24 hours.
Dosage can be gradually increased every 1 to 4 days by 2 mg to 4 mg at each dose based on clinical response and tolerability. The maximum total daily dosage is 36 mg. Single doses greater than 16 mg have not been studied.
There are pharmacokinetic differences when administering Tizanidine between the fed or fasted state[see Clinical Pharmacology ]. Tizanidine may be taken with or without food; however, consistent administration with respect to food is recommended to reduce variability in tizanidine plasma exposure.
Because of the short duration of therapeutic effect, treatment with Tizanidine should be reserved for those daily activities and times when relief of spasticity is most important.,2.3 Recommended Dosage in Patients with Renal ImpairmentIn patients with creatinine clearance < 25 mL/min, use lower individual doses during titration. If higher doses are required, the individual doses rather than dosing frequency should be increased[see Use in Specific Populations and Clinical Pharmacology ].,2.4 Recommended Dosage in Patients with Hepatic ImpairmentIn patients with hepatic impairment, use lower individual doses during titration. If higher doses are required, individual doses rather than dosing frequency should be increased[see Use in Specific Populations and Clinical Pharmacology (12.3)].,2.5 Discontinuation of TizanidineWhen discontinuing Tizanidine, particularly in patients who have been receiving high doses for long periods or who may be on concomitant treatment with narcotics, decrease the dosage by 2 mg to 4 mg per day to minimize the risk of withdrawal adverse reactions[see Drug Abuse andDependence ].) 11/20242.6 Switching Between With/Without Food and Different Tizanidine Dosage FormsThere are pharmacokinetic differences when:
1) switching between administration of Tizanidine with or without food
2) switching between dosage forms if being administered with food.
If these situations occur, monitor patients for therapeutic effect or adverse reactions[see Dosage and Administration and Clinical Pharmacology ]. - Contraindications () 11/20244 CONTRAINDICATIONS
- Concomitant use with strong CYP1A2 inhibitors
- Patients with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine
Tizanidine is contraindicated in patients:
taking strong CYP1A2 inhibitors [see Drug Interactions ].
with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine. Symptoms have included anaphylaxis and angioedema [see Warnings and Precautions ]. - Warnings and Precautions (,5.1 Hypotension
Tizanidine is an α2-adrenergic agonist that can produce hypotension
[see Adverse Reactions and Drug Interactions ]. Syncope has been reported in patients treated with tizanidine in the postmarketing setting. The risk of hypotension may be minimized by dose titration; monitoring for signs and symptoms of hypotension prior to dosage increase may minimize the risks associated with hypotension. In addition, patients moving from a supine to fixed upright position may be at increased risk for hypotension and orthostatic effects.Monitor for hypotension when Tizanidine is used in patients receiving concurrent antihypertensive therapy. It is not recommended that Tizanidine be used with other α2-adrenergic agonists. Clinically significant hypotension (decreases in both systolic and diastolic pressure) has been reported with concomitant administration of tizanidine and strong CYP1A2 inhibitors[seeClinical Pharmacology ]. Therefore, concomitant use of Tizanidine with strong CYP1A2 inhibitors is contraindicated[see Contraindications and Drug Interactions ].].,5.2 Liver InjuryTizanidine may cause hepatocellular liver injury. Liver function test abnormality and hepatotoxicity have been observed with Tizanidine[see Adverse Reactions ]. Monitoring of aminotransferase levels is recommended at baseline and 1 month after maximum dose is achieved, or if hepatic injury is suspected[see Dosage and Administration and Use in Specific Populations ].,5.4 Hallucinosis/Psychotic-Like SymptomsTizanidine use has been associated with hallucinations. Formed, visual hallucinations or delusions were reported in 5 of 170 patients (3%) in two North American controlled clinical studies. Most of the patients were aware that the events were unreal. One patient developed psychosis in association with the hallucinations. One patient among these 5 continued to have problems for at least 2 weeks following discontinuation of tizanidine. Hallucinations have also been reported with tizanidine use in the postmarketing setting. Consider discontinuing Tizanidine in patients who develop hallucinations.) 11/20245.6 Hypersensitivity ReactionsTizanidine can cause anaphylaxis. Signs and symptoms of hypersensitivity, including respiratory compromise, urticaria, and angioedema of the throat and tongue, have been reported. Tizanidine is contraindicated in patients with a history of hypersensitivity reactions to tizanidine[see Contraindications ].
Tizanidine is a central alpha-2-adrenergic agonist indicated for the treatment of spasticity. (
Tizanidine is a central alpha-2-adrenergic agonist indicated for the treatment of spasticity.
Tizanidine is indicated for the treatment of spasticity in adults.
- Monitoring of aminotransferase levels is recommended at baseline and 1 month after maximum dose is achieved. ()2.1 Recommended Evaluation and Testing Before and After Initiating TizanidineMonitoring of aminotransferase levels is recommended at baseline and 1 month after maximum dose is achieved[see Warnings and Precautions ].
- Recommended starting dose: 2 mg by mouth every 6 to 8 hours, as needed, up to a maximum of 3 doses in 24 hours ()2.2 Recommended DosageThe recommended starting dose is 2 mg by mouth every 6 to 8 hours, as needed, to a maximum of three doses in 24 hours.
Dosage can be gradually increased every 1 to 4 days by 2 mg to 4 mg at each dose based on clinical response and tolerability. The maximum total daily dosage is 36 mg. Single doses greater than 16 mg have not been studied.
There are pharmacokinetic differences when administering Tizanidine between the fed or fasted state[see Clinical Pharmacology ]. Tizanidine may be taken with or without food; however, consistent administration with respect to food is recommended to reduce variability in tizanidine plasma exposure.
Because of the short duration of therapeutic effect, treatment with Tizanidine should be reserved for those daily activities and times when relief of spasticity is most important. - Dosage can be increased by 2 mg to 4 mg per dose every 1 to 4 days; maximum total daily dosage is 36 mg ()2.2 Recommended DosageThe recommended starting dose is 2 mg by mouth every 6 to 8 hours, as needed, to a maximum of three doses in 24 hours.
Dosage can be gradually increased every 1 to 4 days by 2 mg to 4 mg at each dose based on clinical response and tolerability. The maximum total daily dosage is 36 mg. Single doses greater than 16 mg have not been studied.
There are pharmacokinetic differences when administering Tizanidine between the fed or fasted state[see Clinical Pharmacology ]. Tizanidine may be taken with or without food; however, consistent administration with respect to food is recommended to reduce variability in tizanidine plasma exposure.
Because of the short duration of therapeutic effect, treatment with Tizanidine should be reserved for those daily activities and times when relief of spasticity is most important. - Tizanidine pharmacokinetics differs between tablets and capsules, and when taken with or without food. These differences could result in a change in tolerability and control of symptoms. Consistent administration with respect to food is recommended. If substitution between dosage forms is necessary, take into consideration these pharmacokinetic differences. (,2.2 Recommended DosageThe recommended starting dose is 2 mg by mouth every 6 to 8 hours, as needed, to a maximum of three doses in 24 hours.
Dosage can be gradually increased every 1 to 4 days by 2 mg to 4 mg at each dose based on clinical response and tolerability. The maximum total daily dosage is 36 mg. Single doses greater than 16 mg have not been studied.
There are pharmacokinetic differences when administering Tizanidine between the fed or fasted state[see Clinical Pharmacology ]. Tizanidine may be taken with or without food; however, consistent administration with respect to food is recommended to reduce variability in tizanidine plasma exposure.
Because of the short duration of therapeutic effect, treatment with Tizanidine should be reserved for those daily activities and times when relief of spasticity is most important.,2.6 Switching Between With/Without Food and Different Tizanidine Dosage FormsThere are pharmacokinetic differences when:
1) switching between administration of Tizanidine with or without food
2) switching between dosage forms if being administered with food.
If these situations occur, monitor patients for therapeutic effect or adverse reactions[see Dosage and Administration and Clinical Pharmacology ].)12.3 PharmacokineticsTizanidine has linear pharmacokinetics over the doses studied in clinical development [1 mg(half the recommended dosage) to 20 mg].
Tizanidine capsules and tablets are bioequivalent to each other under fasting conditions, but not under fed conditions (see Absorption, Effect of Food).
AbsorptionFollowing oral administration, tizanidine is essentially completely absorbed. The absolute oral bioavailability of tizanidine is approximately 40% (CV = 24%), due to extensive first-pass hepatic metabolism.
Effect of Food
There are pharmacokinetic differences between tizanidine capsules and tizanidine tablets with respect to administration with food.
A single dose of either two 4 mg tablets or two 4 mg capsules was administered under fed and fasting conditions in an open-label, four-period, randomized crossover study in 96 volunteers, of whom 81 were eligible for the statistical analysis. Pharmacokinetics under fed conditions were different than under fasting conditions and vary by dosage form.
Tablets or Capsules – Fasting
Tablets – Fed
Capsules – Fed
Capsule Content Sprinkled on Applesauce
Compared to administration of an intact capsule while fasting:
Figure 1: Mean Tizanidine Concentration vs. Time Profiles ForTizanidine Tablets and Capsules (2 × 4 mg) Under Fasted and Fed Conditions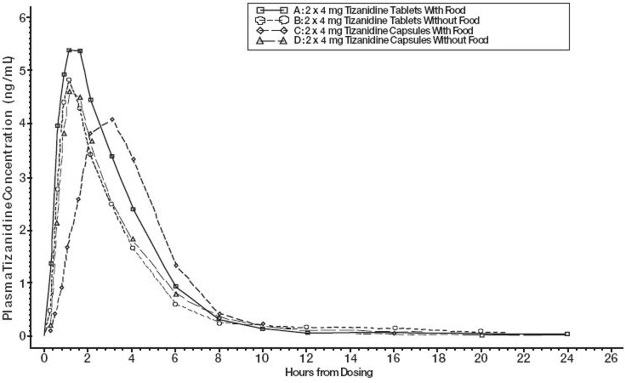 Distribution
DistributionTizanidine is extensively distributed throughout the body with a mean steady state volume of distribution of 2.4 L/kg (CV = 21%) following intravenous administration in healthy adult volunteers. Tizanidine is approximately 30% bound to plasma proteins.
EliminationMetabolismTizanidine has a half-life of approximately 2.5 hours (CV=33%). Approximately 95% of an administered dose is metabolized. The primary cytochrome P450 isoenzyme involved in tizanidine metabolism is CYP1A2. Tizanidine metabolites are not known to be active; their half-lives range from 20 to 40 hours.
ExcretionFollowing single and multiple oral dosing of14C-tizanidine, an average of 60% and 20% of total radioactivity was recovered in the urine and feces, respectively.
Specific PopulationsGeriatric PatientsNo specific pharmacokinetic study was conducted to investigate age effects. Cross study comparison of pharmacokinetic data following single dose administration of 6 mg Tizanidine showed that younger subjects cleared the drug four times faster than the elderly subjects.
[see Use in Specific Populations ].Patients with Hepatic ImpairmentThe influence of hepatic impairment on the pharmacokinetics of tizanidine has not been evaluated. Because tizanidine is extensively metabolized in the liver, hepatic impairment would be expected to have significant effects on pharmacokinetics of tizanidine
[see Use in Specific Populations ].Patients with Renal ImpairmentTizanidine clearance is reduced by more than 50% in elderly patients with renal insufficiency (creatinine clearance < 25 mL/min) compared to healthy elderly subjects; this would be expected to lead to a longer duration of clinical effect
[see Use in Specific Populations ].Gender EffectsNo specific pharmacokinetic study was conducted to investigate gender effects. Retrospective analysis of pharmacokinetic data following single and multiple dose administration of 4 mg Tizanidine, however, showed that gender had no effect on the pharmacokinetics of tizanidine.
Drug InteractionsCYP1A2 InhibitorsThe effects of coadministration of fluvoxamine or ciprofloxacin, both strong CYP1A2 inhibitors, on the pharmacokinetics of a single 4 mg dose of Tizanidine was studied in 10 healthy subjects. The Cmax, AUC, and half-life of tizanidine increased by 12-fold, 33-fold, and 3-fold, respectively, with coadministration of fluvoxamine. The Cmax and AUC of tizanidine increased by 7-fold and 10-fold, respectively, with coadministration of ciprofloxacin
[see Contraindications ].There have been no clinical studies evaluating the effects of other CYP1A2 inhibitors on
tizanidine
[see Drug Interactions ].In vitro studies of cytochrome P450 isoenzymes using human liver microsomes indicate that neither tizanidine nor the major metabolites are likely to affect the metabolism of other drugs metabolized by cytochrome P450 isoenzymes.
Oral ContraceptivesNo specific pharmacokinetic study was conducted to investigate interaction between oral contraceptives and Tizanidine. Retrospective analysis of population pharmacokinetic data following single and multiple dose administration of 4 mg Tizanidine, however, showed that women concurrently taking oral contraceptives had 50% lower clearance of tizanidine compared to women not on oral contraceptives
[see Drug Interactions ].AcetaminophenTizanidine delayed the Tmaxof acetaminophen by 16 minutes. Acetaminophen did not affect the pharmacokinetics of tizanidine.
AlcoholAlcohol increased the AUC and Cmax of tizanidine by approximately 20% and 15%, respectively
[see Drug Interactions ]. - Patients with renal impairment (creatinine clearance <25 mL/min) or hepatic impairment: use lower individual doses during titration. If higher doses are required, individual doses rather than dosing frequency should be increased. (,2.3 Recommended Dosage in Patients with Renal ImpairmentIn patients with creatinine clearance < 25 mL/min, use lower individual doses during titration. If higher doses are required, the individual doses rather than dosing frequency should be increased[see Use in Specific Populations and Clinical Pharmacology ].)2.4 Recommended Dosage in Patients with Hepatic ImpairmentIn patients with hepatic impairment, use lower individual doses during titration. If higher doses are required, individual doses rather than dosing frequency should be increased[see Use in Specific Populations and Clinical Pharmacology (12.3)].
- To discontinue tizanidine, decrease dose slowly to minimize the risk of withdrawal adverse reactions ()2.5 Discontinuation of TizanidineWhen discontinuing Tizanidine, particularly in patients who have been receiving high doses for long periods or who may be on concomitant treatment with narcotics, decrease the dosage by 2 mg to 4 mg per day to minimize the risk of withdrawal adverse reactions[see Drug Abuse andDependence ].
- Tablets 2 mg and 4 mg ()
3 DOSAGE FORMS AND STRENGTHS- Tablets 2 mg and 4 mg
TabletsTizanidine tablets USP, 2 mg are white to off-white, round, flat, bevel edged uncoated tablets debossed with "U" and "168" on one side and bisecting score on other side.
Tizanidine tablets USP, 4 mg are white to off-white, round, flat, bevel edged uncoated tablets debossed with "U" and "169" on one side and quadrisecting score on other side.
● Pregnancy: Based on animal data, may cause fetal harm (
There are no adequate data on the developmental risk associated with use of tizanidine in pregnant women. In animal studies, administration of tizanidine during pregnancy resulted in developmental toxicity (embryofetal and postnatal offspring mortality and growth deficits) at doses less than those used clinically, which were not associated with maternal toxicity (see
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Oral administration of tizanidine (0.3 to 100 mg/kg/day) to pregnant rats during the period of organogenesis resulted in embryofetal and postnatal offspring mortality and reductions in body weight at doses of 30 mg/kg/day and above. Maternal toxicity was observed at the highest dose tested. The no-effect dose for embryofetal developmental toxicity in rats (3 mg/kg/day) is similar to the maximum recommended human dose (MRHD) of 36 mg/day on a body surface area (mg/m2) basis.
Oral administration of tizanidine (1 to 100 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in embryofetal and postnatal offspring mortality at all doses. Maternal toxicity was observed at the highest dose tested. Oral administration of tizanidine (10 and 30 mg/kg/day) during the perinatal period of pregnancy (2-6 days prior to delivery) resulted in increased postnatal offspring mortality at both doses. A no-effect dose for embryofetal developmental toxicity in rabbit was not identified. The lowest dose tested (1 mg/kg/day) is less than the MRHD on a mg/m2basis.
In a pre- and postnatal development study in rats, oral administration of tizanidine (3 to 30 mg/kg/day) resulted in increased postnatal offspring mortality. A no-effect dose for pre- and postnatal developmental toxicity was not identified. The lowest dose tested (3 mg/kg/day) is similar to the MRHD on a mg/m2basis, respectively.
● Geriatric use: Tizanidine should be used with caution in elderly patients because clearance is decreased four-fold (
Tizanidine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Clinical studies of Tizanidine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Pharmacokinetic data showed that younger subjects cleared tizanidine faster than the elderly subjects
- Concomitant use with strong CYP1A2 inhibitors (,4 CONTRAINDICATIONS
- Concomitant use with strong CYP1A2 inhibitors
- Patients with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine
Tizanidine is contraindicated in patients:
taking strong CYP1A2 inhibitors [see Drug Interactions ].
with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine. Symptoms have included anaphylaxis and angioedema [see Warnings and Precautions ].)7.1 Strong CYP1A2 InhibitorsConcomitant use of Tizanidine with strong cytochrome P450 1A2 (CYP1A2) inhibitors (e.g., fluvoxamine, ciprofloxacin) is contraindicated. Changes in pharmacokinetics of tizanidine when administered with a strong CYP1A2 inhibitor resulted in significantly decreased blood pressure, increased drowsiness, and increased psychomotor impairment
[see Contraindications and Clinical Pharmacology ]. - Patients with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine (,4 CONTRAINDICATIONS
- Concomitant use with strong CYP1A2 inhibitors
- Patients with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine
Tizanidine is contraindicated in patients:
taking strong CYP1A2 inhibitors [see Drug Interactions ].
with a history of hypersensitivity to tizanidine or the ingredients in Tizanidine. Symptoms have included anaphylaxis and angioedema [see Warnings and Precautions ].)5.6 Hypersensitivity ReactionsTizanidine can cause anaphylaxis. Signs and symptoms of hypersensitivity, including respiratory compromise, urticaria, and angioedema of the throat and tongue, have been reported. Tizanidine is contraindicated in patients with a history of hypersensitivity reactions to tizanidine[see Contraindications ].