Tobramycin Inhalation
Tobramycin Inhalation Prescribing Information
Warnings and Precautions, Ototoxicity (
Caution should be exercised when prescribing Tobramycin Inhalation Solution to patients with known or suspected auditory or vestibular dysfunction.
Findings related to ototoxicity as measured by audiometric evaluations and auditory adverse event reports were similar between Tobramycin Inhalation Solution and placebo in controlled clinical trials. Hearing loss was reported in two (1.1%) Tobramycin Inhalation Solution-treated patients and in one (0.9%) placebo-treated patient during clinical studies. Additionally, dizziness and vertigo, both of which may be manifestations of vestibular forms Tobramycin Inhalation Solution of ototoxicity, were observed in similar numbers of Tobramycin Inhalation Solution- and placebo-treated patients. Dizziness occurred in two (1.1%) Tobramycin Inhalation Solution-treated patients and one (0.9%) placebo-treated patient and vertigo occurred in two (1.1%) Tobramycin Inhalation Solution-treated patients versus no placebo patients in clinical studies. None of the Tobramycin Inhalation Solution patients discontinued their therapy due to hearing loss, dizziness or vertigo.
Tinnitus may be a sentinel symptom of ototoxicity. No reports of tinnitus occurred in patients during clinical studies with Tobramycin Inhalation Solution, but because it has been observed with inhaled tobramycin solutions
2/2023
Tobramycin Inhalation Solution is indicated for the management of cystic fibrosis patients with
Two, double-blind, randomized, placebo-controlled, parallel group clinical studies (Study 1 and Study 2), which randomized and dosed 306 patients, were conducted in cystic fibrosis patients with
The compressors in the placebo-controlled studies and the bridging study differed from the PARI VIOS compressor to be used with Tobramycin Inhalation Solution. In vitro cascade impaction studies demonstrated that the various compressors used in the clinical trials delivered equivalent doses and respirable fractions of the to-be-marketed Tobramycin Inhalation Solution and TOBI with the marketed compressor (PARI VIOS) when used with the same nebulizer (PARI LC Plus Reusable nebulizer).
All subjects enrolled in both efficacy studies had baseline FEV1% predicted ≥ 40% and ≤ 80% (mean baseline FEV1of 60% of predicted normal) and infected with
Study 1 was a double-blind, single cycle study that randomized 59 patients to receive Tobramycin Inhalation Solution (n=29) or placebo (n=30) for one cycle of treatment (28 days on treatment followed by 28 days off treatment). All patients were ≤ 30 years of age (mean age 12.6 years) and 46% were females. All randomized patients were included in the primary analysis except for one patient who had missing baseline information.
Tobramycin Inhalation Solution significantly improved lung function compared with placebo as measured by the absolute change in FEV1% predicted from baseline to the end of Cycle 1 dosing in the primary analysis population. Treatment with Tobramycin Inhalation Solution and placebo resulted in absolute increases in FEV1% predicted of 16% and 5%, respectively (LS mean difference = 11%; 95% CI: 3, 19; p=0.003). This analysis is adjusted for the covariate of baseline FEV1% predicted, using multiple imputation for missing data. Figure 1 shows the average change in FEV1% predicted over eight weeks.
Study 2 was a randomized, double-blind, 3-cycle, placebo-controlled trial. A total of 247 eligible patients were randomized 2:1 to receive three cycles of Tobramycin Inhalation Solution (n=161) or placebo (n=86). As in Study 1, each cycle comprised 28 days on treatment followed by 28 days off treatment. All patients were ≤46 years of age (mean age 14.8 years) and 44.9% were females. In this study, two randomized patients in the placebo group were not included in the primary efficacy analysis; one withdrew consent without taking any trial medication and the other withdrew due to an adverse drug reaction.
Tobramycin Inhalation Solution significantly improved lung function compared with placebo as measured by the absolute change in FEV1% predicted from baseline to the end of Cycle 3 “ON” period. Treatment with Tobramycin Inhalation Solution and placebo resulted in absolute increases in FEV1% predicted of 7% and 1%, respectively (LS mean difference = 6%; 95% CI: 3, 10; p<0.001). This analysis is adjusted for the covariate of baseline FEV1% predicted, using multiple imputation for missing data. Figure 1 shows the average change in FEV1% predicted over 24 weeks from Study 2.
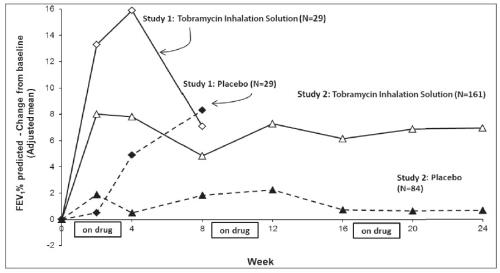
In Study 2, 9.9% of patients treated with Tobramycin Inhalation Solution and 24.7% of patients who received placebo had unplanned hospitalizations due to the disease.
Also in Study 2, 6.2% of patients treated with Tobramycin Inhalation Solution and 16.5% of placebo patients received parenteral tobramycin.

- For oral inhalation only ()2.1 Dosage
Tobramycin Inhalation Solution is for oral inhalation
only [see Dosage and Administration (2.2)]. The recommended dosage of Tobramycin Inhalation Solution for patients six years of age and older is to administer one single-use ampule (300 mg/4 mL) twice daily by oral inhalation in repeated cycles of 28 days on drug, followed by 28 days off drug. The doses should be taken as close to 12 hours apart as possible and not less than 6 hours apart.The 300 mg/4 mL dose of Tobramycin Inhalation Solution is the same for patients regardless of age or weight. Tobramycin Inhalation Solution has not been studied in patients less than six years old.
If patients miss a dose, they should take it as soon as possible anytime up to 6 hours prior to their next scheduled dose. If less than 6 hours remain before the next dose, wait until their next scheduled dose.
- Administer the entire contents of one ampule twice daily by oral inhalation in repeated cycles of 28 days on drug, followed by 28 days off drug. ()2.1 Dosage
Tobramycin Inhalation Solution is for oral inhalation
only [see Dosage and Administration (2.2)]. The recommended dosage of Tobramycin Inhalation Solution for patients six years of age and older is to administer one single-use ampule (300 mg/4 mL) twice daily by oral inhalation in repeated cycles of 28 days on drug, followed by 28 days off drug. The doses should be taken as close to 12 hours apart as possible and not less than 6 hours apart.The 300 mg/4 mL dose of Tobramycin Inhalation Solution is the same for patients regardless of age or weight. Tobramycin Inhalation Solution has not been studied in patients less than six years old.
If patients miss a dose, they should take it as soon as possible anytime up to 6 hours prior to their next scheduled dose. If less than 6 hours remain before the next dose, wait until their next scheduled dose.
Tobramycin Inhalation Solution is supplied as a sterile, clear, colorless to pale yellow, non-pyrogenic, aqueous inhalational solution for nebulization in single-use 4 mL ampule containing 300 mg of tobramycin.
- Aminoglycosides can cause fetal harm when administered to a pregnant woman. ()8.1 PregnancyRisk Summary
Aminoglycosides can cause fetal harm. Published literature reports that use of streptomycin, an aminoglycoside, can cause total, irreversible, bilateral congenital deafness when administered to a pregnant woman
[Warnings and Precautions (5.6)]. Although there are no available data on use of Tobramycin Inhalation Solution in pregnant women to be able to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes, systemic absorption of tobramycin following inhaled administration is expected to be minimal[see Clinical Pharmacology (12.3)]. There are risks to the mother associated with cystic fibrosis in pregnancy(see Clinical Considerations). In animal reproduction studies with subcutaneous administration of tobramycin in pregnant rats and rabbits during organogenesis there were no adverse developmental outcomes; however, ototoxicity was not evaluated in the offspring from these studies(seeData). Advise pregnant women of the potential risk to a fetus.The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical ConsiderationsDisease-Associated Maternal and/or Embryo/Fetal RiskCystic fibrosis may increase the risk for preterm delivery.
DataAnimal DataNo reproduction toxicology studies have been conducted with inhaled tobramycin. However, subcutaneous administration of tobramycin at doses of up to 100 (rat) or 20 (rabbit) mg/kg/day during organogenesis was not associated with adverse developmental outcomes. Subcutaneous doses of tobramycin ≥ 40mg/kg/day were severely maternally toxic to rabbits and precluded the evaluation of adverse developmental outcomes. Ototoxicity was not evaluated in offspring during nonclinical reproductive toxicity studies with tobramycin.
- Nursing mothers: discontinue drug or nursing, taking into consideration the importance of the drug to a mother. ()8.2 LactationRisk Summary
There are no data on the presence of tobramycin in either human or animal milk, the effects on the breastfed infant, or the effects on milk production following oral inhalation of Tobramycin Inhalation Solution. Limited published data on other formulations of tobramycin in lactating women indicate that tobramycin is present in human milk. However, systemic absorption of tobramycin following inhaled administration is expected to be minimal
[see Clinical Pharmacology (12.3)]. Tobramycin may cause alteration in the intestinal flora of the breastfeeding infant(see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Tobramycin Inhalation Solution and any potential adverse effects on the breastfed child from Tobramycin Inhalation Solution or from the underlying maternal condition.Clinical ConsiderationsTobramycin may cause intestinal flora alteration. Advise a woman to monitor the breastfed infant for loose or bloody stools and candidiasis (thrush, diaper rash).
Tobramycin Inhalation Solution is contraindicated in patients with a known hypersensitivity to any aminoglycoside.