Tolcapone Prescribing Information
Tolcapone tablets, USP is indicated as an adjunct to levodopa and carbidopa for the treatment of the signs and symptoms of idiopathic Parkinson's disease. Because of the risk of potentially fatal, acute fulminant liver failure, tolcapone tablets, USP should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa who are experiencing symptom fluctuations and are not responding satisfactorily to or are not appropriate candidates for other adjunctive therapies. Because of the risk of liver injury and because tolcapone tablets, USP, when it is effective, provides an observable symptomatic benefit, the patient who fails to show substantial clinical benefit within 3 weeks of initiation of treatment, should be withdrawn from tolcapone tablets, USP.
The effectiveness of tolcapone tablets, USP was demonstrated in randomized controlled trials in patients receiving concomitant levodopa therapy with carbidopa or another aromatic amino acid decarboxylase inhibitor who experienced end of dose wearing-off phenomena as well as in patients who did not experience such phenomena (see CLINICAL PHARMACOLOGY:
BECAUSE OF THE RISK OF LIVER INJURY AND BECAUSE TOLCAPONE TABLETS WHEN IT IS EFFECTIVE PROVIDES AN OBSERVABLE SYMPTOMATIC BENEFIT, THE PATIENT WHO FAILS TO SHOW SUBSTANTIAL CLINICAL BENEFIT WITHIN 3 WEEKS OF INITIATION OF TREATMENT, SHOULD BE WITHDRAWN FROM TOLCAPONE TABLETS
Patients who develop evidence of hepatocellular injury while on tolcapone tablets and are withdrawn from the drug for any reason may be at increased risk for liver injury if tolcapone tablets is reintroduced. These patients should not ordinarily be considered for retreatment with tolcapone tablets.
Only prescribe tolcapone tablets for patients taking concomitant carbidopa levodopa therapy. The initial dose of tolcapone tablets is always 100 mg three times per day. The recommended daily dose of tolcapone tablets is also 100 mg tid. In clinical trials, elevations in ALT occurred more frequently at the dose of 200 mg tid. While it is unknown whether the risk of acute fulminant liver failure is increased at the 200-mg dose, it would be prudent to use 200 mg only if the anticipated incremental clinical benefit is justified (see BOXED WARNING, WARNINGS, PRECAUTIONS:
In clinical trials, the first dose of the day of tolcapone tablets was always taken together with the first dose of the day of levodopa/carbidopa, and the subsequent doses of tolcapone tablets were given approximately 6 and 12 hours later.
In clinical trials, the majority of patients required a decrease in their daily levodopa dose if their daily dose of levodopa was >600 mg or if patients had moderate or severe dyskinesias before beginning treatment.
To optimize an individual patient's response, reductions in daily levodopa dose may be necessary. In clinical trials, the average reduction in daily levodopa dose was about 30% in those patients requiring a levodopa dose reduction. (Greater than 70% of patients with levodopa doses above 600 mg daily required such a reduction.)
Tolcapone tablets can be combined with both the immediate and sustained release formulations of levodopa/carbidopa.
Tolcapone tablets may be taken with or without food (see CLINICAL PHARMACOLOGY).
BECAUSE OF THE RISK OF LIVER INJURY AND BECAUSE TOLCAPONE TABLETS WHEN IT IS EFFECTIVE PROVIDES AN OBSERVABLE SYMPTOMATIC BENEFIT, THE PATIENT WHO FAILS TO SHOW SUBSTANTIAL CLINICAL BENEFIT WITHIN 3 WEEKS OF INITIATION OF TREATMENT, SHOULD BE WITHDRAWN FROM TOLCAPONE TABLETS
Patients who develop evidence of hepatocellular injury while on tolcapone tablets and are withdrawn from the drug for any reason may be at increased risk for liver injury if tolcapone tablets is reintroduced. These patients should not ordinarily be considered for retreatment with tolcapone tablets.
Only prescribe tolcapone tablets for patients taking concomitant carbidopa levodopa therapy. The initial dose of tolcapone tablets is always 100 mg three times per day. The recommended daily dose of tolcapone tablets is also 100 mg tid. In clinical trials, elevations in ALT occurred more frequently at the dose of 200 mg tid. While it is unknown whether the risk of acute fulminant liver failure is increased at the 200-mg dose, it would be prudent to use 200 mg only if the anticipated incremental clinical benefit is justified (see BOXED WARNING, WARNINGS, PRECAUTIONS:
In clinical trials, the first dose of the day of tolcapone tablets was always taken together with the first dose of the day of levodopa/carbidopa, and the subsequent doses of tolcapone tablets were given approximately 6 and 12 hours later.
In clinical trials, the majority of patients required a decrease in their daily levodopa dose if their daily dose of levodopa was >600 mg or if patients had moderate or severe dyskinesias before beginning treatment.
To optimize an individual patient's response, reductions in daily levodopa dose may be necessary. In clinical trials, the average reduction in daily levodopa dose was about 30% in those patients requiring a levodopa dose reduction. (Greater than 70% of patients with levodopa doses above 600 mg daily required such a reduction.)
Tolcapone tablets can be combined with both the immediate and sustained release formulations of levodopa/carbidopa.
Tolcapone tablets may be taken with or without food (see CLINICAL PHARMACOLOGY).
Tolcapone tablets, USP is indicated as an adjunct to levodopa and carbidopa for the treatment of the signs and symptoms of idiopathic Parkinson's disease. Because of the risk of potentially fatal, acute fulminant liver failure, tolcapone tablets, USP should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa who are experiencing symptom fluctuations and are not responding satisfactorily to or are not appropriate candidates for other adjunctive therapies. Because of the risk of liver injury and because tolcapone tablets, USP, when it is effective, provides an observable symptomatic benefit, the patient who fails to show substantial clinical benefit within 3 weeks of initiation of treatment, should be withdrawn from tolcapone tablets, USP.
The effectiveness of tolcapone tablets, USP was demonstrated in randomized controlled trials in patients receiving concomitant levodopa therapy with carbidopa or another aromatic amino acid decarboxylase inhibitor who experienced end of dose wearing-off phenomena as well as in patients who did not experience such phenomena (see
Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
In mammals, COMT is distributed throughout various organs. The highest activities are in the liver and kidney. COMT also occurs in the heart, lung, smooth and skeletal muscles, intestinal tract, reproductive organs, various glands, adipose tissue, skin, blood cells and neuronal tissues, especially in glial cells. COMT catalyzes the transfer of the methyl group of S-adenosyl-Lmethionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include dopa, catecholamines (dopamine, norepinephrine, epinephrine) and their hydroxylated metabolites. The function of COMT is the elimination of biologically active catechols and some other hydroxylated metabolites. In the presence of a decarboxylase inhibitor, COMT becomes the major metabolizing enzyme for levodopa catalyzing the metabolism to 3-methoxy-4 hydroxy-L-phenylalanine (3-OMD) in the brain and periphery.
The precise mechanism of action of tolcapone is unknown, but it is believed to be related to its ability to inhibit COMT and alter the plasma pharmacokinetics of levodopa. When tolcapone is given in conjunction with levodopa and an aromatic amino acid decarboxylase inhibitor, such as carbidopa, plasma levels of levodopa are more sustained than after administration of levodopa and an aromatic amino acid decarboxylase inhibitor alone. It is believed that these sustained plasma levels of levodopa result in more constant dopaminergic stimulation in the brain, leading to greater effects on the signs and symptoms of Parkinson's disease in patients as well as increased levodopa adverse effects, sometimes requiring a decrease in the dose of levodopa. Tolcapone enters the CNS to a minimal extent, but has been shown to inhibit central COMT activity in animals.
When tolcapone is administered together with levodopa/carbidopa, it increases the relative bioavailability (AUC) of levodopa by approximately twofold. This is due to a decrease in levodopa clearance resulting in a prolongation of the terminal elimination half-life of levodopa (from approximately 2 hours to 3.5 hours). In general, the average peak levodopa plasma concentration (Cmax) and the time of its occurrence (Tmax) are unaffected. The onset of effect occurs after the first administration and is maintained during long-term treatment. Studies in healthy volunteers and Parkinson's disease patients have confirmed that the maximal effect occurs with 100 mg to 200 mg tolcapone. Plasma levels of 3-OMD are markedly and dose-dependently decreased by tolcapone when given with levodopa/carbidopa.
Population pharmacokinetic analyses in patients with Parkinson's disease have shown the same effects of tolcapone on levodopa plasma concentrations that occur in healthy volunteers.
Tolcapone pharmacokinetics are linear over the dose range of 50 mg to 400 mg, independent of levodopa/carbidopa co-administration. The elimination half-life of tolcapone is 2 to 3 hours and there is no significant accumulation. With tid dosing of 100 mg or 200 mg, Cmaxis approximately 3 mcg/mL and 6 mcg/mL, respectively.
Tolcapone pharmacokinetics are independent of sex, age, body weight, and race (Japanese, Black and Caucasian). Polymorphic metabolism is unlikely based on the metabolic pathways involved.
The effectiveness of tolcapone tablets as an adjunct to levodopa in the treatment of Parkinson's disease was established in three multicenter randomized controlled trials of 13 to 26 weeks' duration, supported by four 6-week trials whose results were consistent with those of the longer trials. In two of the longer trials, tolcapone was evaluated in patients whose Parkinson's disease was characterized by deterioration in their response to levodopa at the end of a dosing interval (so-called fluctuating patients with wearing-off phenomena). In the remaining trial, tolcapone was evaluated in patients whose response to levodopa was relatively stable (so-called non- fluctuators).
In addition to the primary outcome, patients were also assessed using sub-parts of the Unified Parkinson's Disease Rating Scale (UPDRS), a frequently used multi-item rating scale intended to evaluate mentation (Part I), activities of daily living (Part II), motor function (Part III), complications of therapy (Part IV), and disease staging (Parts V and VI); an Investigator's Global Assessment of Change (IGA), a subjective scale designed to assess global functioning in 5 areas of Parkinson's disease; the Sickness Impact Profile (SIP), a multi-item scale in 12 domains designed to assess the patient's functioning in multiple areas; and the change in daily levodopa/carbidopa dose.
In one of the studies, 202 patients were randomized in 11 centers in the United States and Canada. In this trial, all patients were receiving concomitant levodopa and carbidopa. In the second trial, 177 patients were randomized in 24 centers in Europe. In this trial, all patients were receiving concomitant levodopa and benserazide.
The following tables display the results of these 2 trials:
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16-hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.2 | -1.2 | --- |
| 100 mg tid | 6.4 | -2.0 | 0.169 |
| 200 mg tid | 5.9 | -3.0 | <0.001 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.7 | 1.4 | --- |
| 100 mg tid | 8.1 | 2.0 | 0.267 |
| 200 mg tid | 9.1 | 2.9 | <0.008 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 948 | 16 | --- |
| 100 mg tid | 788 | -166 | <0.001 |
| 200 mg tid | 865 | -207 | <0.001 |
Global ( overall ) % Improved | |||
| Placebo | --- | 42 | --- |
| 100 mg tid | --- | 71 | <0.001 |
| 200 mg tid | --- | 91 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.5 | -0.4 | --- |
| 100 mg tid | 17.6 | -1.9 | 0.217 |
| 200 mg tid | 20.6 | -2.0 | 0.210 |
UPDRS ADL | |||
| Placebo | 7.5 | -0.3 | --- |
| 100 mg tid | 7.7 | -0.8 | 0.487 |
| 200 mg tid | 8.3 | 0.2 | 0.412 |
SIP ( total ) | |||
| Placebo | 14.7 | -2.2 | --- |
| 100 mg tid | 14.9 | -0.4 | 0.210 |
| 200 mg tid | 17.6 | -0.3 | 0.216 |
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16- hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.1 | -0.7 | --- |
| 100 mg tid | 6.5 | -2.0 | 0.008 |
| 200 mg tid | 6.0 | -1.6 | 0.081 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.5 | -0.1 | --- |
| 100 mg tid | 8.1 | 1.7 | 0.003 |
| 200 mg tid | 8.4 | 1.7 | 0.003 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 660 | -29 | --- |
| 100 mg tid | 667 | -109 | 0.025 |
| 200 mg tid | 675 | -122 | 0.010 |
Global ( overall ) % Improved | |||
| Placebo | --- | 37 | --- |
| 100 mg tid | --- | 70 | 0.003 |
| 200 mg tid | --- | 78 | <0.001 |
UPDRS Motor | |||
| Placebo | 24.0 | -2.1 | --- |
| 100 mg tid | 22.4 | -4.2 | 0.163 |
| 200 mg tid | 22.4 | -6.5 | 0.004 |
UPDRS ADL | |||
| Placebo | 7.9 | -0.5 | --- |
| 100 mg tid | 7.5 | -0.9 | 0.408 |
| 200 mg tid | 7.7 | -1.3 | 0.097 |
SIP ( total ) | |||
| Placebo | 21.6 | -0.9 | --- |
| 100 mg tid | 16.6 | -1.9 | 0.419 |
| 200 mg tid | 18.4 | -4.2 | 0.011 |
Effects on "Off" time and levodopa dose did not differ by age or sex.
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 6 | p - value * | |
UPDRS ADL | |||
| Placebo | 8.5 | 0.1 | --- |
| 100 mg tid | 7.5 | -1.4 | <0.001 |
| 200 mg tid | 7.9 | -1.6 | <0.001 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 6 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 364 | 47 | --- |
| 100 mg tid | 370 | -21 | <0.001 |
| 200 mg tid | 381 | -32 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.7 | 0.1 | --- |
| 100 mg tid | 17.3 | -2.0 | 0.018 |
| 200 mg tid | 16.0 | -2.3 | 0.008 |
SIP ( total ) | |||
| Placebo | 6.9 | 0.4 | --- |
| 100 mg tid | 7.3 | -0.9 | 0.044 |
| 200 mg tid | 7.3 | -0.7 | 0.078 |
Percent of Patients who Developed Fluctuations | |||
| Placebo | --- | 26 | --- |
| 100 mg tid | --- | 19 | 0.297 |
| 200 mg tid | --- | 14 | 0.047 |
Effects on Activities of Daily Living did not differ by age or sex.
Tolcapone tablets, USP is indicated as an adjunct to levodopa and carbidopa for the treatment of the signs and symptoms of idiopathic Parkinson's disease. Because of the risk of potentially fatal, acute fulminant liver failure, tolcapone tablets, USP should ordinarily be used in patients with Parkinson's disease on l-dopa/carbidopa who are experiencing symptom fluctuations and are not responding satisfactorily to or are not appropriate candidates for other adjunctive therapies. Because of the risk of liver injury and because tolcapone tablets, USP, when it is effective, provides an observable symptomatic benefit, the patient who fails to show substantial clinical benefit within 3 weeks of initiation of treatment, should be withdrawn from tolcapone tablets, USP.
The effectiveness of tolcapone tablets, USP was demonstrated in randomized controlled trials in patients receiving concomitant levodopa therapy with carbidopa or another aromatic amino acid decarboxylase inhibitor who experienced end of dose wearing-off phenomena as well as in patients who did not experience such phenomena (see CLINICAL PHARMACOLOGY:
BECAUSE OF THE RISK OF LIVER INJURY AND BECAUSE TOLCAPONE TABLETS WHEN IT IS EFFECTIVE PROVIDES AN OBSERVABLE SYMPTOMATIC BENEFIT, THE PATIENT WHO FAILS TO SHOW SUBSTANTIAL CLINICAL BENEFIT WITHIN 3 WEEKS OF INITIATION OF TREATMENT, SHOULD BE WITHDRAWN FROM TOLCAPONE TABLETS
Patients who develop evidence of hepatocellular injury while on tolcapone tablets and are withdrawn from the drug for any reason may be at increased risk for liver injury if tolcapone tablets is reintroduced. These patients should not ordinarily be considered for retreatment with tolcapone tablets.
Only prescribe tolcapone tablets for patients taking concomitant carbidopa levodopa therapy. The initial dose of tolcapone tablets is always 100 mg three times per day. The recommended daily dose of tolcapone tablets is also 100 mg tid. In clinical trials, elevations in ALT occurred more frequently at the dose of 200 mg tid. While it is unknown whether the risk of acute fulminant liver failure is increased at the 200-mg dose, it would be prudent to use 200 mg only if the anticipated incremental clinical benefit is justified (see BOXED WARNING, WARNINGS, PRECAUTIONS:
In clinical trials, the first dose of the day of tolcapone tablets was always taken together with the first dose of the day of levodopa/carbidopa, and the subsequent doses of tolcapone tablets were given approximately 6 and 12 hours later.
In clinical trials, the majority of patients required a decrease in their daily levodopa dose if their daily dose of levodopa was >600 mg or if patients had moderate or severe dyskinesias before beginning treatment.
To optimize an individual patient's response, reductions in daily levodopa dose may be necessary. In clinical trials, the average reduction in daily levodopa dose was about 30% in those patients requiring a levodopa dose reduction. (Greater than 70% of patients with levodopa doses above 600 mg daily required such a reduction.)
Tolcapone tablets can be combined with both the immediate and sustained release formulations of levodopa/carbidopa.
Tolcapone tablets may be taken with or without food (see CLINICAL PHARMACOLOGY).
BECAUSE OF THE RISK OF LIVER INJURY AND BECAUSE TOLCAPONE TABLETS WHEN IT IS EFFECTIVE PROVIDES AN OBSERVABLE SYMPTOMATIC BENEFIT, THE PATIENT WHO FAILS TO SHOW SUBSTANTIAL CLINICAL BENEFIT WITHIN 3 WEEKS OF INITIATION OF TREATMENT, SHOULD BE WITHDRAWN FROM TOLCAPONE TABLETS
In controlled Phase 3 trials, approximately 5%, 4% and 3% of tolcapone 200 mg tid, 100 mg tid and placebo patients, respectively, reported at least one episode of syncope. Reports of syncope were generally more frequent in patients in all three treatment groups who had an episode of documented hypotension (although the episodes of syncope, obtained by history, were themselves not documented with vital sign measurement) compared to patients who did not have any episodes of documented hypotension.
Typically, diarrhea presents 6 to 12 weeks after tolcapone is started, but it may appear as early as 2 weeks and as late as many months after the initiation of treatment. Clinical trial data suggested that diarrhea associated with tolcapone use may sometimes be associated with anorexia (decreased appetite).
No consistent description of tolcapone-induced diarrhea has been derived from clinical trial data, and the mechanism of action is currently unknown.
It is recommended that all cases of persistent diarrhea should be followed up with an appropriate work-up (including occult blood samples).
In general, hallucinations present shortly after the initiation of therapy with tolcapone (typically within the first 2 weeks). Clinical trial data suggest that hallucinations associated with tolcapone use may be responsive to levodopa dose reduction. Patients whose hallucinations resolved had a mean levodopa dose reduction of 175 mg to 200 mg (20% to 25%) after the onset of the hallucinations. Hallucinations were commonly accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming. The incidence of hallucination may be increased in elderly patients over 75 years treated with tolcapone tablets
Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during tolcapone tablets treatment or after starting or increasing the dose of tolcapone tablets. Other drugs prescribed to improve the symptoms of Parkinson's disease may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with tolcapone tablets because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of tolcapone tablets.
Three cases of pleural effusion, one with pulmonary fibrosis, occurred during clinical trials. These patients were also on concomitant dopamine agonists (pergolide or bromocriptine) and had a prior history of cardiac disease or pulmonary pathology (nonmalignant lung lesion).
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using tolcapone tablets for
Tolcapone tablets should not be used by patients until there has been a complete discussion of the risks and the patient has provided written acknowledgement that the risks have been explained (see PATIENT ACKNOWLEDGEMENT OF RISKSsection).
Inform patients about clinical signs and symptoms that suggest the onset of hepatic injury (persistent nausea, fatigue, lethargy, anorexia, jaundice, dark urine, pruritus, and right upper quadrant tenderness) (see WARNINGS). If symptoms of hepatic failure occur, patients should be advised to contact their physician immediately.
Inform patients of the need to have regular blood tests to monitor liver enzymes.
Advise patients that sleepiness or drowsiness may occur and that they should not drive a car or operate other complex machinery until they have gained sufficient experience on tolcapone tablets to gauge whether or not it adversely affects their mental and/or motor performance. Advise patients to exercise caution while driving, operating machines, or working at heights during treatment with tolcapone tablets. Because of the possible additive sedative effects, caution should also be used when patients are taking other CNS depressants in combination with tolcapone tablets. Inform patients that nausea may occur, especially at the initiation of treatment with tolcapone tablets.
Inform patients that hallucinations and other psychotic-like behavior may occur.
Advise patients about the possibility of developing or worsening of existing dyskinesia and/or dystonia after starting tolcapone tablets.
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Advise patients to rise slowly, especially after long periods of sitting or lying down. Hypotension may be more likely when patients first start treatment with tolcapone tablets.
Instruct patients and caregivers to report intense urges to gamble, increased sexual urges, increase in spending money, binge eating, and other intense urges as well as the inability to control these urges to the prescriber while taking tolcapone tablets.
Although tolcapone tablets has not been shown to be teratogenic in animals, it is always given in conjunction with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in the rabbit. Accordingly, patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy (see PRECAUTIONS:
Tolcapone is excreted into maternal milk in rats. Because of the possibility that tolcapone may be excreted into human milk, advise patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
Before starting treatment with tolcapone tablets, the physician should conduct appropriate tests to exclude the presence of liver disease. In patients determined to be appropriate candidates for treatment with tolcapone tablets, serum glutamic-pyruvic transaminase (SGPT/ALT) and serum glutamic-oxaloacetic transaminase (SGOT/AST) levels should be determined at baseline and periodically (i.e. every 2 to 4 weeks) for the first 6 months of therapy. After the first six months, periodic monitoring is recommended at intervals deemed clinically relevant. Although more frequent monitoring increases the chances of early detection, the precise schedule for monitoring is a matter of clinical judgment.
If the dose is increased to 200 mg tid (see DOSAGE AND ADMINISTRATIONsection), liver enzyme monitoring should take place before increasing the dose and then be conducted every 2 to 4 weeks for the following 6 months of therapy. After six months, periodic monitoring is recommended at intervals deemed clinically relevant.
Due to its affinity to cytochrome P450 2C9 in vitro, tolcapone may interfere with drugs, whose clearance is dependent on this metabolic pathway, such as tolbutamide and warfarin. However, in an in vivo interaction study, tolcapone did not change the pharmacokinetics of tolbutamide. Therefore, clinically relevant interactions involving cytochrome P450 2C9 appear unlikely. Similarly, tolcapone did not affect the pharmacokinetics of desipramine, a drug metabolized by cytochrome P450 2D6, indicating that interactions with drugs metabolized by that enzyme are unlikely. Since clinical information is limited regarding the combination of warfarin and tolcapone, coagulation parameters should be monitored when these two drugs are co-administered.
When tolcapone tablets was given together with levodopa/carbidopa and desipramine, there was no significant change in blood pressure, pulse rate and plasma concentrations of desipramine. Overall, the frequency of adverse events increased slightly. These adverse events were predictable based on the known adverse reactions to each of the three drugs individually. Therefore, caution should be exercised when desipramine is administered to Parkinson's disease patients being treated with tolcapone tablets and levodopa/carbidopa.
In clinical trials, patients receiving tolcapone tablets/levodopa preparations reported a similar adverse event profile independent of whether or not they were also concomitantly administered selegiline (a selective MAO-B inhibitor).
Carcinogenicity studies in which tolcapone was administered in the diet were conducted in mice and rats. Mice were treated for 80 (female) or 95 (male) weeks with doses of 100, 300 and 800 mg/kg/day, equivalent to 0.8, 1.6 and 4 times human exposure (AUC = 80 mcg•hr/mL) at the recommended daily clinical dose of 600 mg. Rats were treated for 104 weeks with doses of 50, 250 and 450 mg/kg/day. Tolcapone exposures were 1, 6.3 and 13 times the human exposure in male rats and 1.7, 11.8 and 26.4 times the human exposure in female rats. There was an increased incidence of uterine adenocarcinomas in female rats at exposure equivalent to 26.4 times the human exposure. There was evidence of renal tubular injury and renal tubular tumor formation in rats. A low incidence of renal tubular cell adenomas occurred in middle- and high-dose female rats; tubular cell carcinomas occurred in middle- and high-dose male and high-dose female rats, with a statistically significant increase in high-dose males. Exposures were equivalent to 6.3 (males) or 11.8 (females) times the human exposure or greater; no renal tumors were observed at exposures of 1 (males) or 1.7 (females) times the human exposure. Minimal-to-marked damage to the renal tubules, consisting of proximal tubule cell degeneration, single cell necrosis, hyperplasia and karyocytomegaly, occurred at the doses associated with renal tumors. Renal tubule damage, characterized by proximal tubule cell degeneration and the presence of atypical nuclei, as well as one adenocarcinoma in a high-dose male, were observed in a 1-year study in rats receiving doses of tolcapone of 150 and 450 mg/kg/day. These histopathological changes suggest the possibility that renal tumor formation might be secondary to chronic cell damage and sustained repair, but this relationship has not been established, and the relevance of these findings to humans is not known. There was no evidence of carcinogenic effects in the long-term mouse study. The carcinogenic potential of tolcapone in combination with levodopa/carbidopa has not been examined.
Tolcapone is always given concomitantly with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in rabbits. The combination of tolcapone (100 mg/kg/day) with levodopa/carbidopa (80/20 mg/kg/day) produced an increased incidence of fetal malformations (primarily external and skeletal digit defects) compared to levodopa/carbidopa alone when pregnant rabbits were treated throughout organogenesis. Plasma exposures to tolcapone (based on AUC) were 0.5 times the expected human exposure, and plasma exposures to levodopa were 6 times higher than those in humans under therapeutic conditions. In a combination embryo-fetal development study in rats, fetal body weights were reduced by the combination of tolcapone (10, 30 and 50 mg/kg/day) and levodopa/carbidopa (120/30 mg/kg/day) and by levodopa/carbidopa alone. Tolcapone exposures were 0.5 times expected human exposure or greater: levodopa exposures were 21 times the expected human exposure or greater. The high dose of 50 mg/kg/day of tolcapone given alone was not associated with reduced fetal body weight (plasma exposures of 1.4 times the expected human exposure).
There is no experience from clinical studies regarding the use of tolcapone tablets in pregnant women. Therefore, tolcapone tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether tolcapone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tolcapone is administered to a nursing woman.
Patients who develop evidence of hepatocellular injury while on tolcapone tablets and are withdrawn from the drug for any reason may be at increased risk for liver injury if tolcapone tablets is reintroduced. These patients should not ordinarily be considered for retreatment with tolcapone tablets.
Only prescribe tolcapone tablets for patients taking concomitant carbidopa levodopa therapy. The initial dose of tolcapone tablets is always 100 mg three times per day. The recommended daily dose of tolcapone tablets is also 100 mg tid. In clinical trials, elevations in ALT occurred more frequently at the dose of 200 mg tid. While it is unknown whether the risk of acute fulminant liver failure is increased at the 200-mg dose, it would be prudent to use 200 mg only if the anticipated incremental clinical benefit is justified (see
In controlled Phase 3 trials, increases to more than 3 times the upper limit of normal in ALT or AST occurred in approximately 1% of patients at 100 mg tid and 3% of patients at 200 mg tid. Females were more likely than males to have an increase in liver enzymes (approximately 5% vs 2%). Approximately one third of patients with elevated enzymes had diarrhea. Increases to more than 8 times the upper limit of normal in liver enzymes occurred in 0.3% at 100 mg tid and 0.7% at 200 mg tid. Elevated enzymes led to discontinuation in 0.3% and 1.7% of patients treated with 100 mg tid and 200 mg tid, respectively. Elevations usually occurred within 6 weeks to 6 months of starting treatment. In about half the cases with elevated liver enzymes, enzyme levels returned to baseline values within 1 to 3 months while patients continued tolcapone tablets treatment. When treatment was discontinued, enzymes generally declined within 2 to 3 weeks but in some cases took as long as 1 to 2 months to return to normal.
Tolcapone can be taken concomitantly with a selective MAO-B inhibitor (e.g., selegiline).
Tolcapone increases plasma levels of levodopa in patients taking concomitant carbidopa levodopa products
The risk for somnolence was increased with tolcapone tablets treatment (tolcapone tablets 100 mg -18 %, 200 mg-14 %, vs placebo-13 %) compared to placebo treatment. In clinical trials, discontinuation due to somnolence occurred in 1 % of patients treated with 200 mg tolcapone tablets and 0 % of patients treated with 100 mg tolcapone tablets or placebo. Falling asleep while engaged in activities of daily living usually occurs in patients experiencing pre-existing somnolence, although some patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness especially since some of the events occur well after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities during treatment with tolcapone tablets.
Before initiating treatment with tolcapone tablets, advise patients about the potential to develop drowsiness and ask specifically about factors that may increase the risk for somnolence with tolcapone tablets such as the use of concomitant sedating medications and the presence of sleep disorders. Consider discontinuing tolcapone tablets in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.). If treatment with tolcapone tablets continues, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
In controlled Phase 3 trials, approximately 5%, 4% and 3% of tolcapone 200 mg tid, 100 mg tid and placebo patients, respectively, reported at least one episode of syncope. Reports of syncope were generally more frequent in patients in all three treatment groups who had an episode of documented hypotension (although the episodes of syncope, obtained by history, were themselves not documented with vital sign measurement) compared to patients who did not have any episodes of documented hypotension.
Typically, diarrhea presents 6 to 12 weeks after tolcapone is started, but it may appear as early as 2 weeks and as late as many months after the initiation of treatment. Clinical trial data suggested that diarrhea associated with tolcapone use may sometimes be associated with anorexia (decreased appetite).
No consistent description of tolcapone-induced diarrhea has been derived from clinical trial data, and the mechanism of action is currently unknown.
It is recommended that all cases of persistent diarrhea should be followed up with an appropriate work-up (including occult blood samples).
In general, hallucinations present shortly after the initiation of therapy with tolcapone (typically within the first 2 weeks). Clinical trial data suggest that hallucinations associated with tolcapone use may be responsive to levodopa dose reduction. Patients whose hallucinations resolved had a mean levodopa dose reduction of 175 mg to 200 mg (20% to 25%) after the onset of the hallucinations. Hallucinations were commonly accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming. The incidence of hallucination may be increased in elderly patients over 75 years treated with tolcapone tablets
Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during tolcapone tablets treatment or after starting or increasing the dose of tolcapone tablets. Other drugs prescribed to improve the symptoms of Parkinson's disease may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with tolcapone tablets because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of tolcapone tablets.
Three cases of pleural effusion, one with pulmonary fibrosis, occurred during clinical trials. These patients were also on concomitant dopamine agonists (pergolide or bromocriptine) and had a prior history of cardiac disease or pulmonary pathology (nonmalignant lung lesion).
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using tolcapone tablets for
Tolcapone tablets should not be used by patients until there has been a complete discussion of the risks and the patient has provided written acknowledgement that the risks have been explained (see PATIENT ACKNOWLEDGEMENT OF RISKSsection).
Inform patients about clinical signs and symptoms that suggest the onset of hepatic injury (persistent nausea, fatigue, lethargy, anorexia, jaundice, dark urine, pruritus, and right upper quadrant tenderness) (see WARNINGS). If symptoms of hepatic failure occur, patients should be advised to contact their physician immediately.
Inform patients of the need to have regular blood tests to monitor liver enzymes.
Advise patients that sleepiness or drowsiness may occur and that they should not drive a car or operate other complex machinery until they have gained sufficient experience on tolcapone tablets to gauge whether or not it adversely affects their mental and/or motor performance. Advise patients to exercise caution while driving, operating machines, or working at heights during treatment with tolcapone tablets. Because of the possible additive sedative effects, caution should also be used when patients are taking other CNS depressants in combination with tolcapone tablets. Inform patients that nausea may occur, especially at the initiation of treatment with tolcapone tablets.
Inform patients that hallucinations and other psychotic-like behavior may occur.
Advise patients about the possibility of developing or worsening of existing dyskinesia and/or dystonia after starting tolcapone tablets.
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Advise patients to rise slowly, especially after long periods of sitting or lying down. Hypotension may be more likely when patients first start treatment with tolcapone tablets.
Instruct patients and caregivers to report intense urges to gamble, increased sexual urges, increase in spending money, binge eating, and other intense urges as well as the inability to control these urges to the prescriber while taking tolcapone tablets.
Although tolcapone tablets has not been shown to be teratogenic in animals, it is always given in conjunction with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in the rabbit. Accordingly, patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy (see PRECAUTIONS:
Tolcapone is excreted into maternal milk in rats. Because of the possibility that tolcapone may be excreted into human milk, advise patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
Before starting treatment with tolcapone tablets, the physician should conduct appropriate tests to exclude the presence of liver disease. In patients determined to be appropriate candidates for treatment with tolcapone tablets, serum glutamic-pyruvic transaminase (SGPT/ALT) and serum glutamic-oxaloacetic transaminase (SGOT/AST) levels should be determined at baseline and periodically (i.e. every 2 to 4 weeks) for the first 6 months of therapy. After the first six months, periodic monitoring is recommended at intervals deemed clinically relevant. Although more frequent monitoring increases the chances of early detection, the precise schedule for monitoring is a matter of clinical judgment.
If the dose is increased to 200 mg tid (see DOSAGE AND ADMINISTRATIONsection), liver enzyme monitoring should take place before increasing the dose and then be conducted every 2 to 4 weeks for the following 6 months of therapy. After six months, periodic monitoring is recommended at intervals deemed clinically relevant.
Due to its affinity to cytochrome P450 2C9 in vitro, tolcapone may interfere with drugs, whose clearance is dependent on this metabolic pathway, such as tolbutamide and warfarin. However, in an in vivo interaction study, tolcapone did not change the pharmacokinetics of tolbutamide. Therefore, clinically relevant interactions involving cytochrome P450 2C9 appear unlikely. Similarly, tolcapone did not affect the pharmacokinetics of desipramine, a drug metabolized by cytochrome P450 2D6, indicating that interactions with drugs metabolized by that enzyme are unlikely. Since clinical information is limited regarding the combination of warfarin and tolcapone, coagulation parameters should be monitored when these two drugs are co-administered.
When tolcapone tablets was given together with levodopa/carbidopa and desipramine, there was no significant change in blood pressure, pulse rate and plasma concentrations of desipramine. Overall, the frequency of adverse events increased slightly. These adverse events were predictable based on the known adverse reactions to each of the three drugs individually. Therefore, caution should be exercised when desipramine is administered to Parkinson's disease patients being treated with tolcapone tablets and levodopa/carbidopa.
In clinical trials, patients receiving tolcapone tablets/levodopa preparations reported a similar adverse event profile independent of whether or not they were also concomitantly administered selegiline (a selective MAO-B inhibitor).
Carcinogenicity studies in which tolcapone was administered in the diet were conducted in mice and rats. Mice were treated for 80 (female) or 95 (male) weeks with doses of 100, 300 and 800 mg/kg/day, equivalent to 0.8, 1.6 and 4 times human exposure (AUC = 80 mcg•hr/mL) at the recommended daily clinical dose of 600 mg. Rats were treated for 104 weeks with doses of 50, 250 and 450 mg/kg/day. Tolcapone exposures were 1, 6.3 and 13 times the human exposure in male rats and 1.7, 11.8 and 26.4 times the human exposure in female rats. There was an increased incidence of uterine adenocarcinomas in female rats at exposure equivalent to 26.4 times the human exposure. There was evidence of renal tubular injury and renal tubular tumor formation in rats. A low incidence of renal tubular cell adenomas occurred in middle- and high-dose female rats; tubular cell carcinomas occurred in middle- and high-dose male and high-dose female rats, with a statistically significant increase in high-dose males. Exposures were equivalent to 6.3 (males) or 11.8 (females) times the human exposure or greater; no renal tumors were observed at exposures of 1 (males) or 1.7 (females) times the human exposure. Minimal-to-marked damage to the renal tubules, consisting of proximal tubule cell degeneration, single cell necrosis, hyperplasia and karyocytomegaly, occurred at the doses associated with renal tumors. Renal tubule damage, characterized by proximal tubule cell degeneration and the presence of atypical nuclei, as well as one adenocarcinoma in a high-dose male, were observed in a 1-year study in rats receiving doses of tolcapone of 150 and 450 mg/kg/day. These histopathological changes suggest the possibility that renal tumor formation might be secondary to chronic cell damage and sustained repair, but this relationship has not been established, and the relevance of these findings to humans is not known. There was no evidence of carcinogenic effects in the long-term mouse study. The carcinogenic potential of tolcapone in combination with levodopa/carbidopa has not been examined.
Tolcapone is always given concomitantly with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in rabbits. The combination of tolcapone (100 mg/kg/day) with levodopa/carbidopa (80/20 mg/kg/day) produced an increased incidence of fetal malformations (primarily external and skeletal digit defects) compared to levodopa/carbidopa alone when pregnant rabbits were treated throughout organogenesis. Plasma exposures to tolcapone (based on AUC) were 0.5 times the expected human exposure, and plasma exposures to levodopa were 6 times higher than those in humans under therapeutic conditions. In a combination embryo-fetal development study in rats, fetal body weights were reduced by the combination of tolcapone (10, 30 and 50 mg/kg/day) and levodopa/carbidopa (120/30 mg/kg/day) and by levodopa/carbidopa alone. Tolcapone exposures were 0.5 times expected human exposure or greater: levodopa exposures were 21 times the expected human exposure or greater. The high dose of 50 mg/kg/day of tolcapone given alone was not associated with reduced fetal body weight (plasma exposures of 1.4 times the expected human exposure).
There is no experience from clinical studies regarding the use of tolcapone tablets in pregnant women. Therefore, tolcapone tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether tolcapone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tolcapone is administered to a nursing woman.
In clinical trials, the first dose of the day of tolcapone tablets was always taken together with the first dose of the day of levodopa/carbidopa, and the subsequent doses of tolcapone tablets were given approximately 6 and 12 hours later.
In clinical trials, the majority of patients required a decrease in their daily levodopa dose if their daily dose of levodopa was >600 mg or if patients had moderate or severe dyskinesias before beginning treatment.
To optimize an individual patient's response, reductions in daily levodopa dose may be necessary. In clinical trials, the average reduction in daily levodopa dose was about 30% in those patients requiring a levodopa dose reduction. (Greater than 70% of patients with levodopa doses above 600 mg daily required such a reduction.)
Tolcapone tablets can be combined with both the immediate and sustained release formulations of levodopa/carbidopa.
Tolcapone tablets may be taken with or without food (see
Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
In mammals, COMT is distributed throughout various organs. The highest activities are in the liver and kidney. COMT also occurs in the heart, lung, smooth and skeletal muscles, intestinal tract, reproductive organs, various glands, adipose tissue, skin, blood cells and neuronal tissues, especially in glial cells. COMT catalyzes the transfer of the methyl group of S-adenosyl-Lmethionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include dopa, catecholamines (dopamine, norepinephrine, epinephrine) and their hydroxylated metabolites. The function of COMT is the elimination of biologically active catechols and some other hydroxylated metabolites. In the presence of a decarboxylase inhibitor, COMT becomes the major metabolizing enzyme for levodopa catalyzing the metabolism to 3-methoxy-4 hydroxy-L-phenylalanine (3-OMD) in the brain and periphery.
The precise mechanism of action of tolcapone is unknown, but it is believed to be related to its ability to inhibit COMT and alter the plasma pharmacokinetics of levodopa. When tolcapone is given in conjunction with levodopa and an aromatic amino acid decarboxylase inhibitor, such as carbidopa, plasma levels of levodopa are more sustained than after administration of levodopa and an aromatic amino acid decarboxylase inhibitor alone. It is believed that these sustained plasma levels of levodopa result in more constant dopaminergic stimulation in the brain, leading to greater effects on the signs and symptoms of Parkinson's disease in patients as well as increased levodopa adverse effects, sometimes requiring a decrease in the dose of levodopa. Tolcapone enters the CNS to a minimal extent, but has been shown to inhibit central COMT activity in animals.
When tolcapone is administered together with levodopa/carbidopa, it increases the relative bioavailability (AUC) of levodopa by approximately twofold. This is due to a decrease in levodopa clearance resulting in a prolongation of the terminal elimination half-life of levodopa (from approximately 2 hours to 3.5 hours). In general, the average peak levodopa plasma concentration (Cmax) and the time of its occurrence (Tmax) are unaffected. The onset of effect occurs after the first administration and is maintained during long-term treatment. Studies in healthy volunteers and Parkinson's disease patients have confirmed that the maximal effect occurs with 100 mg to 200 mg tolcapone. Plasma levels of 3-OMD are markedly and dose-dependently decreased by tolcapone when given with levodopa/carbidopa.
Population pharmacokinetic analyses in patients with Parkinson's disease have shown the same effects of tolcapone on levodopa plasma concentrations that occur in healthy volunteers.
Tolcapone pharmacokinetics are linear over the dose range of 50 mg to 400 mg, independent of levodopa/carbidopa co-administration. The elimination half-life of tolcapone is 2 to 3 hours and there is no significant accumulation. With tid dosing of 100 mg or 200 mg, Cmaxis approximately 3 mcg/mL and 6 mcg/mL, respectively.
Tolcapone pharmacokinetics are independent of sex, age, body weight, and race (Japanese, Black and Caucasian). Polymorphic metabolism is unlikely based on the metabolic pathways involved.
The effectiveness of tolcapone tablets as an adjunct to levodopa in the treatment of Parkinson's disease was established in three multicenter randomized controlled trials of 13 to 26 weeks' duration, supported by four 6-week trials whose results were consistent with those of the longer trials. In two of the longer trials, tolcapone was evaluated in patients whose Parkinson's disease was characterized by deterioration in their response to levodopa at the end of a dosing interval (so-called fluctuating patients with wearing-off phenomena). In the remaining trial, tolcapone was evaluated in patients whose response to levodopa was relatively stable (so-called non- fluctuators).
In addition to the primary outcome, patients were also assessed using sub-parts of the Unified Parkinson's Disease Rating Scale (UPDRS), a frequently used multi-item rating scale intended to evaluate mentation (Part I), activities of daily living (Part II), motor function (Part III), complications of therapy (Part IV), and disease staging (Parts V and VI); an Investigator's Global Assessment of Change (IGA), a subjective scale designed to assess global functioning in 5 areas of Parkinson's disease; the Sickness Impact Profile (SIP), a multi-item scale in 12 domains designed to assess the patient's functioning in multiple areas; and the change in daily levodopa/carbidopa dose.
In one of the studies, 202 patients were randomized in 11 centers in the United States and Canada. In this trial, all patients were receiving concomitant levodopa and carbidopa. In the second trial, 177 patients were randomized in 24 centers in Europe. In this trial, all patients were receiving concomitant levodopa and benserazide.
The following tables display the results of these 2 trials:
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16-hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.2 | -1.2 | --- |
| 100 mg tid | 6.4 | -2.0 | 0.169 |
| 200 mg tid | 5.9 | -3.0 | <0.001 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.7 | 1.4 | --- |
| 100 mg tid | 8.1 | 2.0 | 0.267 |
| 200 mg tid | 9.1 | 2.9 | <0.008 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 948 | 16 | --- |
| 100 mg tid | 788 | -166 | <0.001 |
| 200 mg tid | 865 | -207 | <0.001 |
Global ( overall ) % Improved | |||
| Placebo | --- | 42 | --- |
| 100 mg tid | --- | 71 | <0.001 |
| 200 mg tid | --- | 91 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.5 | -0.4 | --- |
| 100 mg tid | 17.6 | -1.9 | 0.217 |
| 200 mg tid | 20.6 | -2.0 | 0.210 |
UPDRS ADL | |||
| Placebo | 7.5 | -0.3 | --- |
| 100 mg tid | 7.7 | -0.8 | 0.487 |
| 200 mg tid | 8.3 | 0.2 | 0.412 |
SIP ( total ) | |||
| Placebo | 14.7 | -2.2 | --- |
| 100 mg tid | 14.9 | -0.4 | 0.210 |
| 200 mg tid | 17.6 | -0.3 | 0.216 |
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16- hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.1 | -0.7 | --- |
| 100 mg tid | 6.5 | -2.0 | 0.008 |
| 200 mg tid | 6.0 | -1.6 | 0.081 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.5 | -0.1 | --- |
| 100 mg tid | 8.1 | 1.7 | 0.003 |
| 200 mg tid | 8.4 | 1.7 | 0.003 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 660 | -29 | --- |
| 100 mg tid | 667 | -109 | 0.025 |
| 200 mg tid | 675 | -122 | 0.010 |
Global ( overall ) % Improved | |||
| Placebo | --- | 37 | --- |
| 100 mg tid | --- | 70 | 0.003 |
| 200 mg tid | --- | 78 | <0.001 |
UPDRS Motor | |||
| Placebo | 24.0 | -2.1 | --- |
| 100 mg tid | 22.4 | -4.2 | 0.163 |
| 200 mg tid | 22.4 | -6.5 | 0.004 |
UPDRS ADL | |||
| Placebo | 7.9 | -0.5 | --- |
| 100 mg tid | 7.5 | -0.9 | 0.408 |
| 200 mg tid | 7.7 | -1.3 | 0.097 |
SIP ( total ) | |||
| Placebo | 21.6 | -0.9 | --- |
| 100 mg tid | 16.6 | -1.9 | 0.419 |
| 200 mg tid | 18.4 | -4.2 | 0.011 |
Effects on "Off" time and levodopa dose did not differ by age or sex.
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 6 | p - value * | |
UPDRS ADL | |||
| Placebo | 8.5 | 0.1 | --- |
| 100 mg tid | 7.5 | -1.4 | <0.001 |
| 200 mg tid | 7.9 | -1.6 | <0.001 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 6 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 364 | 47 | --- |
| 100 mg tid | 370 | -21 | <0.001 |
| 200 mg tid | 381 | -32 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.7 | 0.1 | --- |
| 100 mg tid | 17.3 | -2.0 | 0.018 |
| 200 mg tid | 16.0 | -2.3 | 0.008 |
SIP ( total ) | |||
| Placebo | 6.9 | 0.4 | --- |
| 100 mg tid | 7.3 | -0.9 | 0.044 |
| 200 mg tid | 7.3 | -0.7 | 0.078 |
Percent of Patients who Developed Fluctuations | |||
| Placebo | --- | 26 | --- |
| 100 mg tid | --- | 19 | 0.297 |
| 200 mg tid | --- | 14 | 0.047 |
Effects on Activities of Daily Living did not differ by age or sex.
In controlled Phase 3 trials, increases to more than 3 times the upper limit of normal in ALT or AST occurred in approximately 1% of patients at 100 mg tid and 3% of patients at 200 mg tid. Females were more likely than males to have an increase in liver enzymes (approximately 5% vs 2%). Approximately one third of patients with elevated enzymes had diarrhea. Increases to more than 8 times the upper limit of normal in liver enzymes occurred in 0.3% at 100 mg tid and 0.7% at 200 mg tid. Elevated enzymes led to discontinuation in 0.3% and 1.7% of patients treated with 100 mg tid and 200 mg tid, respectively. Elevations usually occurred within 6 weeks to 6 months of starting treatment. In about half the cases with elevated liver enzymes, enzyme levels returned to baseline values within 1 to 3 months while patients continued tolcapone tablets treatment. When treatment was discontinued, enzymes generally declined within 2 to 3 weeks but in some cases took as long as 1 to 2 months to return to normal.
Tolcapone can be taken concomitantly with a selective MAO-B inhibitor (e.g., selegiline).
Tolcapone increases plasma levels of levodopa in patients taking concomitant carbidopa levodopa products
The risk for somnolence was increased with tolcapone tablets treatment (tolcapone tablets 100 mg -18 %, 200 mg-14 %, vs placebo-13 %) compared to placebo treatment. In clinical trials, discontinuation due to somnolence occurred in 1 % of patients treated with 200 mg tolcapone tablets and 0 % of patients treated with 100 mg tolcapone tablets or placebo. Falling asleep while engaged in activities of daily living usually occurs in patients experiencing pre-existing somnolence, although some patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness especially since some of the events occur well after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities during treatment with tolcapone tablets.
Before initiating treatment with tolcapone tablets, advise patients about the potential to develop drowsiness and ask specifically about factors that may increase the risk for somnolence with tolcapone tablets such as the use of concomitant sedating medications and the presence of sleep disorders. Consider discontinuing tolcapone tablets in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.). If treatment with tolcapone tablets continues, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if patients become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
In mammals, COMT is distributed throughout various organs. The highest activities are in the liver and kidney. COMT also occurs in the heart, lung, smooth and skeletal muscles, intestinal tract, reproductive organs, various glands, adipose tissue, skin, blood cells and neuronal tissues, especially in glial cells. COMT catalyzes the transfer of the methyl group of S-adenosyl-Lmethionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include dopa, catecholamines (dopamine, norepinephrine, epinephrine) and their hydroxylated metabolites. The function of COMT is the elimination of biologically active catechols and some other hydroxylated metabolites. In the presence of a decarboxylase inhibitor, COMT becomes the major metabolizing enzyme for levodopa catalyzing the metabolism to 3-methoxy-4 hydroxy-L-phenylalanine (3-OMD) in the brain and periphery.
The precise mechanism of action of tolcapone is unknown, but it is believed to be related to its ability to inhibit COMT and alter the plasma pharmacokinetics of levodopa. When tolcapone is given in conjunction with levodopa and an aromatic amino acid decarboxylase inhibitor, such as carbidopa, plasma levels of levodopa are more sustained than after administration of levodopa and an aromatic amino acid decarboxylase inhibitor alone. It is believed that these sustained plasma levels of levodopa result in more constant dopaminergic stimulation in the brain, leading to greater effects on the signs and symptoms of Parkinson's disease in patients as well as increased levodopa adverse effects, sometimes requiring a decrease in the dose of levodopa. Tolcapone enters the CNS to a minimal extent, but has been shown to inhibit central COMT activity in animals.
When tolcapone is administered together with levodopa/carbidopa, it increases the relative bioavailability (AUC) of levodopa by approximately twofold. This is due to a decrease in levodopa clearance resulting in a prolongation of the terminal elimination half-life of levodopa (from approximately 2 hours to 3.5 hours). In general, the average peak levodopa plasma concentration (Cmax) and the time of its occurrence (Tmax) are unaffected. The onset of effect occurs after the first administration and is maintained during long-term treatment. Studies in healthy volunteers and Parkinson's disease patients have confirmed that the maximal effect occurs with 100 mg to 200 mg tolcapone. Plasma levels of 3-OMD are markedly and dose-dependently decreased by tolcapone when given with levodopa/carbidopa.
Population pharmacokinetic analyses in patients with Parkinson's disease have shown the same effects of tolcapone on levodopa plasma concentrations that occur in healthy volunteers.
Tolcapone pharmacokinetics are linear over the dose range of 50 mg to 400 mg, independent of levodopa/carbidopa co-administration. The elimination half-life of tolcapone is 2 to 3 hours and there is no significant accumulation. With tid dosing of 100 mg or 200 mg, Cmaxis approximately 3 mcg/mL and 6 mcg/mL, respectively.
Tolcapone pharmacokinetics are independent of sex, age, body weight, and race (Japanese, Black and Caucasian). Polymorphic metabolism is unlikely based on the metabolic pathways involved.
The effectiveness of tolcapone tablets as an adjunct to levodopa in the treatment of Parkinson's disease was established in three multicenter randomized controlled trials of 13 to 26 weeks' duration, supported by four 6-week trials whose results were consistent with those of the longer trials. In two of the longer trials, tolcapone was evaluated in patients whose Parkinson's disease was characterized by deterioration in their response to levodopa at the end of a dosing interval (so-called fluctuating patients with wearing-off phenomena). In the remaining trial, tolcapone was evaluated in patients whose response to levodopa was relatively stable (so-called non- fluctuators).
In addition to the primary outcome, patients were also assessed using sub-parts of the Unified Parkinson's Disease Rating Scale (UPDRS), a frequently used multi-item rating scale intended to evaluate mentation (Part I), activities of daily living (Part II), motor function (Part III), complications of therapy (Part IV), and disease staging (Parts V and VI); an Investigator's Global Assessment of Change (IGA), a subjective scale designed to assess global functioning in 5 areas of Parkinson's disease; the Sickness Impact Profile (SIP), a multi-item scale in 12 domains designed to assess the patient's functioning in multiple areas; and the change in daily levodopa/carbidopa dose.
In one of the studies, 202 patients were randomized in 11 centers in the United States and Canada. In this trial, all patients were receiving concomitant levodopa and carbidopa. In the second trial, 177 patients were randomized in 24 centers in Europe. In this trial, all patients were receiving concomitant levodopa and benserazide.
The following tables display the results of these 2 trials:
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16-hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.2 | -1.2 | --- |
| 100 mg tid | 6.4 | -2.0 | 0.169 |
| 200 mg tid | 5.9 | -3.0 | <0.001 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.7 | 1.4 | --- |
| 100 mg tid | 8.1 | 2.0 | 0.267 |
| 200 mg tid | 9.1 | 2.9 | <0.008 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 948 | 16 | --- |
| 100 mg tid | 788 | -166 | <0.001 |
| 200 mg tid | 865 | -207 | <0.001 |
Global ( overall ) % Improved | |||
| Placebo | --- | 42 | --- |
| 100 mg tid | --- | 71 | <0.001 |
| 200 mg tid | --- | 91 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.5 | -0.4 | --- |
| 100 mg tid | 17.6 | -1.9 | 0.217 |
| 200 mg tid | 20.6 | -2.0 | 0.210 |
UPDRS ADL | |||
| Placebo | 7.5 | -0.3 | --- |
| 100 mg tid | 7.7 | -0.8 | 0.487 |
| 200 mg tid | 8.3 | 0.2 | 0.412 |
SIP ( total ) | |||
| Placebo | 14.7 | -2.2 | --- |
| 100 mg tid | 14.9 | -0.4 | 0.210 |
| 200 mg tid | 17.6 | -0.3 | 0.216 |
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16- hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.1 | -0.7 | --- |
| 100 mg tid | 6.5 | -2.0 | 0.008 |
| 200 mg tid | 6.0 | -1.6 | 0.081 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.5 | -0.1 | --- |
| 100 mg tid | 8.1 | 1.7 | 0.003 |
| 200 mg tid | 8.4 | 1.7 | 0.003 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 660 | -29 | --- |
| 100 mg tid | 667 | -109 | 0.025 |
| 200 mg tid | 675 | -122 | 0.010 |
Global ( overall ) % Improved | |||
| Placebo | --- | 37 | --- |
| 100 mg tid | --- | 70 | 0.003 |
| 200 mg tid | --- | 78 | <0.001 |
UPDRS Motor | |||
| Placebo | 24.0 | -2.1 | --- |
| 100 mg tid | 22.4 | -4.2 | 0.163 |
| 200 mg tid | 22.4 | -6.5 | 0.004 |
UPDRS ADL | |||
| Placebo | 7.9 | -0.5 | --- |
| 100 mg tid | 7.5 | -0.9 | 0.408 |
| 200 mg tid | 7.7 | -1.3 | 0.097 |
SIP ( total ) | |||
| Placebo | 21.6 | -0.9 | --- |
| 100 mg tid | 16.6 | -1.9 | 0.419 |
| 200 mg tid | 18.4 | -4.2 | 0.011 |
Effects on "Off" time and levodopa dose did not differ by age or sex.
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 6 | p - value * | |
UPDRS ADL | |||
| Placebo | 8.5 | 0.1 | --- |
| 100 mg tid | 7.5 | -1.4 | <0.001 |
| 200 mg tid | 7.9 | -1.6 | <0.001 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 6 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 364 | 47 | --- |
| 100 mg tid | 370 | -21 | <0.001 |
| 200 mg tid | 381 | -32 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.7 | 0.1 | --- |
| 100 mg tid | 17.3 | -2.0 | 0.018 |
| 200 mg tid | 16.0 | -2.3 | 0.008 |
SIP ( total ) | |||
| Placebo | 6.9 | 0.4 | --- |
| 100 mg tid | 7.3 | -0.9 | 0.044 |
| 200 mg tid | 7.3 | -0.7 | 0.078 |
Percent of Patients who Developed Fluctuations | |||
| Placebo | --- | 26 | --- |
| 100 mg tid | --- | 19 | 0.297 |
| 200 mg tid | --- | 14 | 0.047 |
Effects on Activities of Daily Living did not differ by age or sex.
Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
In mammals, COMT is distributed throughout various organs. The highest activities are in the liver and kidney. COMT also occurs in the heart, lung, smooth and skeletal muscles, intestinal tract, reproductive organs, various glands, adipose tissue, skin, blood cells and neuronal tissues, especially in glial cells. COMT catalyzes the transfer of the methyl group of S-adenosyl-Lmethionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include dopa, catecholamines (dopamine, norepinephrine, epinephrine) and their hydroxylated metabolites. The function of COMT is the elimination of biologically active catechols and some other hydroxylated metabolites. In the presence of a decarboxylase inhibitor, COMT becomes the major metabolizing enzyme for levodopa catalyzing the metabolism to 3-methoxy-4 hydroxy-L-phenylalanine (3-OMD) in the brain and periphery.
The precise mechanism of action of tolcapone is unknown, but it is believed to be related to its ability to inhibit COMT and alter the plasma pharmacokinetics of levodopa. When tolcapone is given in conjunction with levodopa and an aromatic amino acid decarboxylase inhibitor, such as carbidopa, plasma levels of levodopa are more sustained than after administration of levodopa and an aromatic amino acid decarboxylase inhibitor alone. It is believed that these sustained plasma levels of levodopa result in more constant dopaminergic stimulation in the brain, leading to greater effects on the signs and symptoms of Parkinson's disease in patients as well as increased levodopa adverse effects, sometimes requiring a decrease in the dose of levodopa. Tolcapone enters the CNS to a minimal extent, but has been shown to inhibit central COMT activity in animals.
When tolcapone is administered together with levodopa/carbidopa, it increases the relative bioavailability (AUC) of levodopa by approximately twofold. This is due to a decrease in levodopa clearance resulting in a prolongation of the terminal elimination half-life of levodopa (from approximately 2 hours to 3.5 hours). In general, the average peak levodopa plasma concentration (Cmax) and the time of its occurrence (Tmax) are unaffected. The onset of effect occurs after the first administration and is maintained during long-term treatment. Studies in healthy volunteers and Parkinson's disease patients have confirmed that the maximal effect occurs with 100 mg to 200 mg tolcapone. Plasma levels of 3-OMD are markedly and dose-dependently decreased by tolcapone when given with levodopa/carbidopa.
Population pharmacokinetic analyses in patients with Parkinson's disease have shown the same effects of tolcapone on levodopa plasma concentrations that occur in healthy volunteers.
Tolcapone pharmacokinetics are linear over the dose range of 50 mg to 400 mg, independent of levodopa/carbidopa co-administration. The elimination half-life of tolcapone is 2 to 3 hours and there is no significant accumulation. With tid dosing of 100 mg or 200 mg, Cmaxis approximately 3 mcg/mL and 6 mcg/mL, respectively.
Tolcapone pharmacokinetics are independent of sex, age, body weight, and race (Japanese, Black and Caucasian). Polymorphic metabolism is unlikely based on the metabolic pathways involved.
The effectiveness of tolcapone tablets as an adjunct to levodopa in the treatment of Parkinson's disease was established in three multicenter randomized controlled trials of 13 to 26 weeks' duration, supported by four 6-week trials whose results were consistent with those of the longer trials. In two of the longer trials, tolcapone was evaluated in patients whose Parkinson's disease was characterized by deterioration in their response to levodopa at the end of a dosing interval (so-called fluctuating patients with wearing-off phenomena). In the remaining trial, tolcapone was evaluated in patients whose response to levodopa was relatively stable (so-called non- fluctuators).
In addition to the primary outcome, patients were also assessed using sub-parts of the Unified Parkinson's Disease Rating Scale (UPDRS), a frequently used multi-item rating scale intended to evaluate mentation (Part I), activities of daily living (Part II), motor function (Part III), complications of therapy (Part IV), and disease staging (Parts V and VI); an Investigator's Global Assessment of Change (IGA), a subjective scale designed to assess global functioning in 5 areas of Parkinson's disease; the Sickness Impact Profile (SIP), a multi-item scale in 12 domains designed to assess the patient's functioning in multiple areas; and the change in daily levodopa/carbidopa dose.
In one of the studies, 202 patients were randomized in 11 centers in the United States and Canada. In this trial, all patients were receiving concomitant levodopa and carbidopa. In the second trial, 177 patients were randomized in 24 centers in Europe. In this trial, all patients were receiving concomitant levodopa and benserazide.
The following tables display the results of these 2 trials:
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16-hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.2 | -1.2 | --- |
| 100 mg tid | 6.4 | -2.0 | 0.169 |
| 200 mg tid | 5.9 | -3.0 | <0.001 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.7 | 1.4 | --- |
| 100 mg tid | 8.1 | 2.0 | 0.267 |
| 200 mg tid | 9.1 | 2.9 | <0.008 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 948 | 16 | --- |
| 100 mg tid | 788 | -166 | <0.001 |
| 200 mg tid | 865 | -207 | <0.001 |
Global ( overall ) % Improved | |||
| Placebo | --- | 42 | --- |
| 100 mg tid | --- | 71 | <0.001 |
| 200 mg tid | --- | 91 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.5 | -0.4 | --- |
| 100 mg tid | 17.6 | -1.9 | 0.217 |
| 200 mg tid | 20.6 | -2.0 | 0.210 |
UPDRS ADL | |||
| Placebo | 7.5 | -0.3 | --- |
| 100 mg tid | 7.7 | -0.8 | 0.487 |
| 200 mg tid | 8.3 | 0.2 | 0.412 |
SIP ( total ) | |||
| Placebo | 14.7 | -2.2 | --- |
| 100 mg tid | 14.9 | -0.4 | 0.210 |
| 200 mg tid | 17.6 | -0.3 | 0.216 |
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
** Hours “Off” or “On” are based on the percent of waking day “Off” or “On”, assuming a 16- hour waking day. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 3 ( hrs ) | p - value * | |
Hours of Wake Time “ Off ” ** | |||
| Placebo | 6.1 | -0.7 | --- |
| 100 mg tid | 6.5 | -2.0 | 0.008 |
| 200 mg tid | 6.0 | -1.6 | 0.081 |
Hours of Wake Time “ On ” ** | |||
| Placebo | 8.5 | -0.1 | --- |
| 100 mg tid | 8.1 | 1.7 | 0.003 |
| 200 mg tid | 8.4 | 1.7 | 0.003 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 3 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 660 | -29 | --- |
| 100 mg tid | 667 | -109 | 0.025 |
| 200 mg tid | 675 | -122 | 0.010 |
Global ( overall ) % Improved | |||
| Placebo | --- | 37 | --- |
| 100 mg tid | --- | 70 | 0.003 |
| 200 mg tid | --- | 78 | <0.001 |
UPDRS Motor | |||
| Placebo | 24.0 | -2.1 | --- |
| 100 mg tid | 22.4 | -4.2 | 0.163 |
| 200 mg tid | 22.4 | -6.5 | 0.004 |
UPDRS ADL | |||
| Placebo | 7.9 | -0.5 | --- |
| 100 mg tid | 7.5 | -0.9 | 0.408 |
| 200 mg tid | 7.7 | -1.3 | 0.097 |
SIP ( total ) | |||
| Placebo | 21.6 | -0.9 | --- |
| 100 mg tid | 16.6 | -1.9 | 0.419 |
| 200 mg tid | 18.4 | -4.2 | 0.011 |
Effects on "Off" time and levodopa dose did not differ by age or sex.
* Compared to placebo. Nominal p values are not adjusted for multiple comparisons. | |||
Primary Measure | |||
Baseline ( hrs ) | Change from Baseline at Month 6 | p - value * | |
UPDRS ADL | |||
| Placebo | 8.5 | 0.1 | --- |
| 100 mg tid | 7.5 | -1.4 | <0.001 |
| 200 mg tid | 7.9 | -1.6 | <0.001 |
Secondary Measure | |||
Baseline | Change from Baseline at Month 6 | p - value * | |
Levodopa Total Daily Dose ( mg ) | |||
| Placebo | 364 | 47 | --- |
| 100 mg tid | 370 | -21 | <0.001 |
| 200 mg tid | 381 | -32 | <0.001 |
UPDRS Motor | |||
| Placebo | 19.7 | 0.1 | --- |
| 100 mg tid | 17.3 | -2.0 | 0.018 |
| 200 mg tid | 16.0 | -2.3 | 0.008 |
SIP ( total ) | |||
| Placebo | 6.9 | 0.4 | --- |
| 100 mg tid | 7.3 | -0.9 | 0.044 |
| 200 mg tid | 7.3 | -0.7 | 0.078 |
Percent of Patients who Developed Fluctuations | |||
| Placebo | --- | 26 | --- |
| 100 mg tid | --- | 19 | 0.297 |
| 200 mg tid | --- | 14 | 0.047 |
Effects on Activities of Daily Living did not differ by age or sex.
In controlled Phase 3 trials, approximately 5%, 4% and 3% of tolcapone 200 mg tid, 100 mg tid and placebo patients, respectively, reported at least one episode of syncope. Reports of syncope were generally more frequent in patients in all three treatment groups who had an episode of documented hypotension (although the episodes of syncope, obtained by history, were themselves not documented with vital sign measurement) compared to patients who did not have any episodes of documented hypotension.
Typically, diarrhea presents 6 to 12 weeks after tolcapone is started, but it may appear as early as 2 weeks and as late as many months after the initiation of treatment. Clinical trial data suggested that diarrhea associated with tolcapone use may sometimes be associated with anorexia (decreased appetite).
No consistent description of tolcapone-induced diarrhea has been derived from clinical trial data, and the mechanism of action is currently unknown.
It is recommended that all cases of persistent diarrhea should be followed up with an appropriate work-up (including occult blood samples).
In general, hallucinations present shortly after the initiation of therapy with tolcapone (typically within the first 2 weeks). Clinical trial data suggest that hallucinations associated with tolcapone use may be responsive to levodopa dose reduction. Patients whose hallucinations resolved had a mean levodopa dose reduction of 175 mg to 200 mg (20% to 25%) after the onset of the hallucinations. Hallucinations were commonly accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming. The incidence of hallucination may be increased in elderly patients over 75 years treated with tolcapone tablets
Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during tolcapone tablets treatment or after starting or increasing the dose of tolcapone tablets. Other drugs prescribed to improve the symptoms of Parkinson's disease may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with tolcapone tablets because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of tolcapone tablets.
Three cases of pleural effusion, one with pulmonary fibrosis, occurred during clinical trials. These patients were also on concomitant dopamine agonists (pergolide or bromocriptine) and had a prior history of cardiac disease or pulmonary pathology (nonmalignant lung lesion).
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using tolcapone tablets for
Tolcapone tablets should not be used by patients until there has been a complete discussion of the risks and the patient has provided written acknowledgement that the risks have been explained (see PATIENT ACKNOWLEDGEMENT OF RISKSsection).
Inform patients about clinical signs and symptoms that suggest the onset of hepatic injury (persistent nausea, fatigue, lethargy, anorexia, jaundice, dark urine, pruritus, and right upper quadrant tenderness) (see WARNINGS). If symptoms of hepatic failure occur, patients should be advised to contact their physician immediately.
Inform patients of the need to have regular blood tests to monitor liver enzymes.
Advise patients that sleepiness or drowsiness may occur and that they should not drive a car or operate other complex machinery until they have gained sufficient experience on tolcapone tablets to gauge whether or not it adversely affects their mental and/or motor performance. Advise patients to exercise caution while driving, operating machines, or working at heights during treatment with tolcapone tablets. Because of the possible additive sedative effects, caution should also be used when patients are taking other CNS depressants in combination with tolcapone tablets. Inform patients that nausea may occur, especially at the initiation of treatment with tolcapone tablets.
Inform patients that hallucinations and other psychotic-like behavior may occur.
Advise patients about the possibility of developing or worsening of existing dyskinesia and/or dystonia after starting tolcapone tablets.
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Advise patients to rise slowly, especially after long periods of sitting or lying down. Hypotension may be more likely when patients first start treatment with tolcapone tablets.
Instruct patients and caregivers to report intense urges to gamble, increased sexual urges, increase in spending money, binge eating, and other intense urges as well as the inability to control these urges to the prescriber while taking tolcapone tablets.
Although tolcapone tablets has not been shown to be teratogenic in animals, it is always given in conjunction with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in the rabbit. Accordingly, patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy (see PRECAUTIONS:
Tolcapone is excreted into maternal milk in rats. Because of the possibility that tolcapone may be excreted into human milk, advise patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
Before starting treatment with tolcapone tablets, the physician should conduct appropriate tests to exclude the presence of liver disease. In patients determined to be appropriate candidates for treatment with tolcapone tablets, serum glutamic-pyruvic transaminase (SGPT/ALT) and serum glutamic-oxaloacetic transaminase (SGOT/AST) levels should be determined at baseline and periodically (i.e. every 2 to 4 weeks) for the first 6 months of therapy. After the first six months, periodic monitoring is recommended at intervals deemed clinically relevant. Although more frequent monitoring increases the chances of early detection, the precise schedule for monitoring is a matter of clinical judgment.
If the dose is increased to 200 mg tid (see DOSAGE AND ADMINISTRATIONsection), liver enzyme monitoring should take place before increasing the dose and then be conducted every 2 to 4 weeks for the following 6 months of therapy. After six months, periodic monitoring is recommended at intervals deemed clinically relevant.
Due to its affinity to cytochrome P450 2C9 in vitro, tolcapone may interfere with drugs, whose clearance is dependent on this metabolic pathway, such as tolbutamide and warfarin. However, in an in vivo interaction study, tolcapone did not change the pharmacokinetics of tolbutamide. Therefore, clinically relevant interactions involving cytochrome P450 2C9 appear unlikely. Similarly, tolcapone did not affect the pharmacokinetics of desipramine, a drug metabolized by cytochrome P450 2D6, indicating that interactions with drugs metabolized by that enzyme are unlikely. Since clinical information is limited regarding the combination of warfarin and tolcapone, coagulation parameters should be monitored when these two drugs are co-administered.
When tolcapone tablets was given together with levodopa/carbidopa and desipramine, there was no significant change in blood pressure, pulse rate and plasma concentrations of desipramine. Overall, the frequency of adverse events increased slightly. These adverse events were predictable based on the known adverse reactions to each of the three drugs individually. Therefore, caution should be exercised when desipramine is administered to Parkinson's disease patients being treated with tolcapone tablets and levodopa/carbidopa.
In clinical trials, patients receiving tolcapone tablets/levodopa preparations reported a similar adverse event profile independent of whether or not they were also concomitantly administered selegiline (a selective MAO-B inhibitor).
Carcinogenicity studies in which tolcapone was administered in the diet were conducted in mice and rats. Mice were treated for 80 (female) or 95 (male) weeks with doses of 100, 300 and 800 mg/kg/day, equivalent to 0.8, 1.6 and 4 times human exposure (AUC = 80 mcg•hr/mL) at the recommended daily clinical dose of 600 mg. Rats were treated for 104 weeks with doses of 50, 250 and 450 mg/kg/day. Tolcapone exposures were 1, 6.3 and 13 times the human exposure in male rats and 1.7, 11.8 and 26.4 times the human exposure in female rats. There was an increased incidence of uterine adenocarcinomas in female rats at exposure equivalent to 26.4 times the human exposure. There was evidence of renal tubular injury and renal tubular tumor formation in rats. A low incidence of renal tubular cell adenomas occurred in middle- and high-dose female rats; tubular cell carcinomas occurred in middle- and high-dose male and high-dose female rats, with a statistically significant increase in high-dose males. Exposures were equivalent to 6.3 (males) or 11.8 (females) times the human exposure or greater; no renal tumors were observed at exposures of 1 (males) or 1.7 (females) times the human exposure. Minimal-to-marked damage to the renal tubules, consisting of proximal tubule cell degeneration, single cell necrosis, hyperplasia and karyocytomegaly, occurred at the doses associated with renal tumors. Renal tubule damage, characterized by proximal tubule cell degeneration and the presence of atypical nuclei, as well as one adenocarcinoma in a high-dose male, were observed in a 1-year study in rats receiving doses of tolcapone of 150 and 450 mg/kg/day. These histopathological changes suggest the possibility that renal tumor formation might be secondary to chronic cell damage and sustained repair, but this relationship has not been established, and the relevance of these findings to humans is not known. There was no evidence of carcinogenic effects in the long-term mouse study. The carcinogenic potential of tolcapone in combination with levodopa/carbidopa has not been examined.
Tolcapone is always given concomitantly with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in rabbits. The combination of tolcapone (100 mg/kg/day) with levodopa/carbidopa (80/20 mg/kg/day) produced an increased incidence of fetal malformations (primarily external and skeletal digit defects) compared to levodopa/carbidopa alone when pregnant rabbits were treated throughout organogenesis. Plasma exposures to tolcapone (based on AUC) were 0.5 times the expected human exposure, and plasma exposures to levodopa were 6 times higher than those in humans under therapeutic conditions. In a combination embryo-fetal development study in rats, fetal body weights were reduced by the combination of tolcapone (10, 30 and 50 mg/kg/day) and levodopa/carbidopa (120/30 mg/kg/day) and by levodopa/carbidopa alone. Tolcapone exposures were 0.5 times expected human exposure or greater: levodopa exposures were 21 times the expected human exposure or greater. The high dose of 50 mg/kg/day of tolcapone given alone was not associated with reduced fetal body weight (plasma exposures of 1.4 times the expected human exposure).
There is no experience from clinical studies regarding the use of tolcapone tablets in pregnant women. Therefore, tolcapone tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether tolcapone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tolcapone is administered to a nursing woman.
Tolcapone tablets are contraindicated in patients with liver disease, in patients who were withdrawn from tolcapone tablets because of evidence of tolcapone tablets-induced hepatocellular injury or who have demonstrated hypersensitivity to the drug or its ingredients.
Tolcapone tablets is also contraindicated in patients with a history of nontraumatic rhabdomyolysis or hyperpyrexia and confusion possibly related to medication (see
In controlled Phase 3 trials, approximately 5%, 4% and 3% of tolcapone 200 mg tid, 100 mg tid and placebo patients, respectively, reported at least one episode of syncope. Reports of syncope were generally more frequent in patients in all three treatment groups who had an episode of documented hypotension (although the episodes of syncope, obtained by history, were themselves not documented with vital sign measurement) compared to patients who did not have any episodes of documented hypotension.
Typically, diarrhea presents 6 to 12 weeks after tolcapone is started, but it may appear as early as 2 weeks and as late as many months after the initiation of treatment. Clinical trial data suggested that diarrhea associated with tolcapone use may sometimes be associated with anorexia (decreased appetite).
No consistent description of tolcapone-induced diarrhea has been derived from clinical trial data, and the mechanism of action is currently unknown.
It is recommended that all cases of persistent diarrhea should be followed up with an appropriate work-up (including occult blood samples).
In general, hallucinations present shortly after the initiation of therapy with tolcapone (typically within the first 2 weeks). Clinical trial data suggest that hallucinations associated with tolcapone use may be responsive to levodopa dose reduction. Patients whose hallucinations resolved had a mean levodopa dose reduction of 175 mg to 200 mg (20% to 25%) after the onset of the hallucinations. Hallucinations were commonly accompanied by confusion and to a lesser extent sleep disorder (insomnia) and excessive dreaming. The incidence of hallucination may be increased in elderly patients over 75 years treated with tolcapone tablets
Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during tolcapone tablets treatment or after starting or increasing the dose of tolcapone tablets. Other drugs prescribed to improve the symptoms of Parkinson's disease may have similar effects on thinking and behavior. This abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
Ordinarily, patients with a major psychotic disorder should not be treated with tolcapone tablets because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of tolcapone tablets.
Three cases of pleural effusion, one with pulmonary fibrosis, occurred during clinical trials. These patients were also on concomitant dopamine agonists (pergolide or bromocriptine) and had a prior history of cardiac disease or pulmonary pathology (nonmalignant lung lesion).
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using tolcapone tablets for
Tolcapone tablets should not be used by patients until there has been a complete discussion of the risks and the patient has provided written acknowledgement that the risks have been explained (see PATIENT ACKNOWLEDGEMENT OF RISKSsection).
Inform patients about clinical signs and symptoms that suggest the onset of hepatic injury (persistent nausea, fatigue, lethargy, anorexia, jaundice, dark urine, pruritus, and right upper quadrant tenderness) (see WARNINGS). If symptoms of hepatic failure occur, patients should be advised to contact their physician immediately.
Inform patients of the need to have regular blood tests to monitor liver enzymes.
Advise patients that sleepiness or drowsiness may occur and that they should not drive a car or operate other complex machinery until they have gained sufficient experience on tolcapone tablets to gauge whether or not it adversely affects their mental and/or motor performance. Advise patients to exercise caution while driving, operating machines, or working at heights during treatment with tolcapone tablets. Because of the possible additive sedative effects, caution should also be used when patients are taking other CNS depressants in combination with tolcapone tablets. Inform patients that nausea may occur, especially at the initiation of treatment with tolcapone tablets.
Inform patients that hallucinations and other psychotic-like behavior may occur.
Advise patients about the possibility of developing or worsening of existing dyskinesia and/or dystonia after starting tolcapone tablets.
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Advise patients to rise slowly, especially after long periods of sitting or lying down. Hypotension may be more likely when patients first start treatment with tolcapone tablets.
Instruct patients and caregivers to report intense urges to gamble, increased sexual urges, increase in spending money, binge eating, and other intense urges as well as the inability to control these urges to the prescriber while taking tolcapone tablets.
Although tolcapone tablets has not been shown to be teratogenic in animals, it is always given in conjunction with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in the rabbit. Accordingly, patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy (see PRECAUTIONS:
Tolcapone is excreted into maternal milk in rats. Because of the possibility that tolcapone may be excreted into human milk, advise patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant.
Before starting treatment with tolcapone tablets, the physician should conduct appropriate tests to exclude the presence of liver disease. In patients determined to be appropriate candidates for treatment with tolcapone tablets, serum glutamic-pyruvic transaminase (SGPT/ALT) and serum glutamic-oxaloacetic transaminase (SGOT/AST) levels should be determined at baseline and periodically (i.e. every 2 to 4 weeks) for the first 6 months of therapy. After the first six months, periodic monitoring is recommended at intervals deemed clinically relevant. Although more frequent monitoring increases the chances of early detection, the precise schedule for monitoring is a matter of clinical judgment.
If the dose is increased to 200 mg tid (see DOSAGE AND ADMINISTRATIONsection), liver enzyme monitoring should take place before increasing the dose and then be conducted every 2 to 4 weeks for the following 6 months of therapy. After six months, periodic monitoring is recommended at intervals deemed clinically relevant.
Due to its affinity to cytochrome P450 2C9 in vitro, tolcapone may interfere with drugs, whose clearance is dependent on this metabolic pathway, such as tolbutamide and warfarin. However, in an in vivo interaction study, tolcapone did not change the pharmacokinetics of tolbutamide. Therefore, clinically relevant interactions involving cytochrome P450 2C9 appear unlikely. Similarly, tolcapone did not affect the pharmacokinetics of desipramine, a drug metabolized by cytochrome P450 2D6, indicating that interactions with drugs metabolized by that enzyme are unlikely. Since clinical information is limited regarding the combination of warfarin and tolcapone, coagulation parameters should be monitored when these two drugs are co-administered.
When tolcapone tablets was given together with levodopa/carbidopa and desipramine, there was no significant change in blood pressure, pulse rate and plasma concentrations of desipramine. Overall, the frequency of adverse events increased slightly. These adverse events were predictable based on the known adverse reactions to each of the three drugs individually. Therefore, caution should be exercised when desipramine is administered to Parkinson's disease patients being treated with tolcapone tablets and levodopa/carbidopa.
In clinical trials, patients receiving tolcapone tablets/levodopa preparations reported a similar adverse event profile independent of whether or not they were also concomitantly administered selegiline (a selective MAO-B inhibitor).
Carcinogenicity studies in which tolcapone was administered in the diet were conducted in mice and rats. Mice were treated for 80 (female) or 95 (male) weeks with doses of 100, 300 and 800 mg/kg/day, equivalent to 0.8, 1.6 and 4 times human exposure (AUC = 80 mcg•hr/mL) at the recommended daily clinical dose of 600 mg. Rats were treated for 104 weeks with doses of 50, 250 and 450 mg/kg/day. Tolcapone exposures were 1, 6.3 and 13 times the human exposure in male rats and 1.7, 11.8 and 26.4 times the human exposure in female rats. There was an increased incidence of uterine adenocarcinomas in female rats at exposure equivalent to 26.4 times the human exposure. There was evidence of renal tubular injury and renal tubular tumor formation in rats. A low incidence of renal tubular cell adenomas occurred in middle- and high-dose female rats; tubular cell carcinomas occurred in middle- and high-dose male and high-dose female rats, with a statistically significant increase in high-dose males. Exposures were equivalent to 6.3 (males) or 11.8 (females) times the human exposure or greater; no renal tumors were observed at exposures of 1 (males) or 1.7 (females) times the human exposure. Minimal-to-marked damage to the renal tubules, consisting of proximal tubule cell degeneration, single cell necrosis, hyperplasia and karyocytomegaly, occurred at the doses associated with renal tumors. Renal tubule damage, characterized by proximal tubule cell degeneration and the presence of atypical nuclei, as well as one adenocarcinoma in a high-dose male, were observed in a 1-year study in rats receiving doses of tolcapone of 150 and 450 mg/kg/day. These histopathological changes suggest the possibility that renal tumor formation might be secondary to chronic cell damage and sustained repair, but this relationship has not been established, and the relevance of these findings to humans is not known. There was no evidence of carcinogenic effects in the long-term mouse study. The carcinogenic potential of tolcapone in combination with levodopa/carbidopa has not been examined.
Tolcapone is always given concomitantly with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in rabbits. The combination of tolcapone (100 mg/kg/day) with levodopa/carbidopa (80/20 mg/kg/day) produced an increased incidence of fetal malformations (primarily external and skeletal digit defects) compared to levodopa/carbidopa alone when pregnant rabbits were treated throughout organogenesis. Plasma exposures to tolcapone (based on AUC) were 0.5 times the expected human exposure, and plasma exposures to levodopa were 6 times higher than those in humans under therapeutic conditions. In a combination embryo-fetal development study in rats, fetal body weights were reduced by the combination of tolcapone (10, 30 and 50 mg/kg/day) and levodopa/carbidopa (120/30 mg/kg/day) and by levodopa/carbidopa alone. Tolcapone exposures were 0.5 times expected human exposure or greater: levodopa exposures were 21 times the expected human exposure or greater. The high dose of 50 mg/kg/day of tolcapone given alone was not associated with reduced fetal body weight (plasma exposures of 1.4 times the expected human exposure).
There is no experience from clinical studies regarding the use of tolcapone tablets in pregnant women. Therefore, tolcapone tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
It is not known whether tolcapone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tolcapone is administered to a nursing woman.
The imprecision of the estimated increase is due to uncertainties about the base rate and the actual number of cases occurring in association with tolcapone tablets. The incidence of idiopathic potentially fatal fulminant hepatic failure (i.e., not due to viral hepatitis or alcohol) is low. One estimate, based upon transplant registry data, is approximately 3/1,000,000 patients per year in the United States. Whether this estimate is an appropriate basis for estimating the increased risk of liver failure among tolcapone tablets users is uncertain. Tolcapone tablets users, for example, differ in age and general health status from candidates for liver transplantation. Similarly, underreporting of cases may lead to significant underestimation of the increased risk associated with the use of tolcapone tablets.
During the premarketing development of tolcapone, two distinct patient populations were studied, patients with end-of-dose wearing-off phenomena and patients with stable responses to levodopa therapy. All patients received concomitant treatment with levodopa preparations, however, and were similar in other clinical aspects. Adverse reactions are shown for these two populations combined.
The most commonly observed adverse reactions in the double-blind, placebo-controlled trials (N=892), with a difference in incidence (tolcapone tablets minus Placebo) of at least 5 % or greater in the 100 mg or 200 mg tolcapone tablets- treated groups compared to placebo, were dyskinesia, nausea, diarrhea, anorexia, sleep disorder, vomiting, urine discoloration, somnolence, hallucination, dystonia, and sweating.
Approximately 16% of the 592 patients who participated in the double-blind, placebo- controlled trials discontinued treatment due to adverse reactions compared to 10% of the 298 patients who received placebo. Diarrhea was by far the most frequent cause of discontinuation (approximately 6% in tolcapone patients vs. 1% on placebo).
Table 4 lists treatment emergent adverse reactions that occurred in at least 1% of patients treated with tolcapone participating in the double-blind, placebo-controlled studies and were numerically more common in at least one of the tolcapone groups. In these studies, either tolcapone or placebo was added to levodopa/carbidopa (or benserazide).
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse reactions in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical studies. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. However, the cited figures do provide the prescriber with some basis for estimating the relative contribution of drug and nondrug factors to the adverse reactions incidence rate in the population studied.
Placebo | Tolcapone tid | ||
N = 298 | 100 mg N = 296 | 200 mg N = 298 | |
| Adverse Reactions | (%) | (%) | (%) |
| Dyskinesia | 20 | 42 | 51 |
| Nausea | 18 | 30 | 35 |
| Sleep Disorder | 18 | 24 | 25 |
| Dystonia | 17 | 19 | 22 |
| Dreaming Excessive | 17 | 21 | 16 |
| Anorexia | 13 | 19 | 23 |
| Cramps Muscle | 17 | 17 | 18 |
| Orthostatic Complaints | 14 | 17 | 17 |
| Somnolence | 13 | 18 | 14 |
| Diarrhea | 8 | 16 | 18 |
| Confusion | 9 | 11 | 10 |
| Dizziness | 10 | 13 | 6 |
| Headache | 7 | 10 | 11 |
| Hallucination | 5 | 8 | 10 |
| Vomiting | 4 | 8 | 10 |
| Constipation | 5 | 6 | 8 |
| Fatigue | 6 | 7 | 3 |
| Upper Respiratory Tract Infection | 3 | 5 | 7 |
| Falling | 4 | 4 | 6 |
| Sweating Increased | 2 | 4 | 7 |
| Urinary Tract Infection | 4 | 5 | 5 |
| Xerostomia | 2 | 5 | 6 |
| Abdominal Pain | 3 | 5 | 6 |
| Syncope | 3 | 4 | 5 |
| Urine Discoloration | 1 | 2 | 7 |
| Dyspepsia | 2 | 4 | 3 |
| Influenza | 2 | 3 | 4 |
| Dyspnea | 2 | 3 | 3 |
| Balance Loss | 2 | 3 | 2 |
| Flatulence | 2 | 2 | 4 |
| Hyperkinesia | 1 | 3 | 2 |
| Chest Pain | 1 | 3 | 1 |
| Hypotension | 1 | 2 | 2 |
| Paresthesia | 2 | 3 | 1 |
| Stiffness | 1 | 2 | 2 |
| Arthritis | 1 | 2 | 1 |
| Chest Discomfort | 1 | 1 | 2 |
| Hypokinesia | 1 | 1 | 3 |
| Micturition Disorder | 1 | 2 | 1 |
| Pain Neck | 1 | 2 | 2 |
| Burning | 0 | 2 | 1 |
| Sinus Congestion | 0 | 2 | 1 |
| Agitation | 0 | 1 | 1 |
| Bleeding Dermal | 0 | 1 | 1 |
| Irritability | 0 | 1 | 1 |
| Mental Deficiency | 0 | 1 | 1 |
| Hyperactivity | 0 | 1 | 1 |
| Malaise | 0 | 1 | 0 |
| Panic Reaction | 0 | 1 | 0 |
| Tumor Skin | 0 | 1 | 0 |
| Cataract | 0 | 1 | 0 |
| Euphoria | 0 | 1 | 0 |
| Fever | 0 | 0 | 1 |
| Alopecia | 0 | 1 | 0 |
| Eye Inflamed | 0 | 1 | 0 |
| Hyertonia | 0 | 0 | 1 |
| Tumor Uterus | 0 | 1 | 0 |
During these trials, all adverse events were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse events, similar types of adverse events were grouped into a smaller number of standardized categories using COSTART dictionary terminology. These categories are used in the listing below.
All reported events that occurred at least twice (or once for serious or potentially serious events), except those already listed above, trivial events and terms too vague to be meaningful are included, without regard to determination of a causal relationship to tolcapone tablets.
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are defined as those occurring in between 1/100 and 1/1000 patients; and rare adverse events are defined as those occurring in fewer than 1/1000 patients.
Tolcapone tablets, USP is available as tablets containing 100 mg tolcapone, USP.
Tolcapone, an inhibitor of catechol-O-methyltransferase (COMT), is used in the treatment of Parkinson's disease as an adjunct to levodopa/carbidopa therapy. It is a yellow, non-hygroscopic, fine powder or fine powder with lumps with a relative molecular mass of 273.24. The chemical name of tolcapone is 3,4-dihydroxy-4'-methyl-5-nitrobenzophenone. Its empirical formula is C14H11NO5 and its structural formula is:
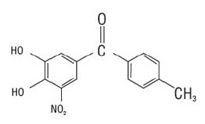
Inactive ingredients: Core: lactose monohydrate, microcrystalline cellulose, dibasic calcium phosphate anhydrous, sodium starch glycolate, povidone, talc and magnesium stearate.Film coating: hydroxypropyl methylcellulose, titanium dioxide, talc, ethyl cellulose, triacetin,and sodium lauryl sulfate, with the following dye system: yellow iron oxide.